A-Tap™: Improving The Moment That Matters Most
The A-Tap™ Product Story
Progress in medicine doesn’t always come from discovering new drugs or therapies. Sometimes, it comes from improving mundane tasks repeated every day in clinical practice worldwide.
During clinical drug trials, a Canadian pharmaceutical company kept running into the same problem.
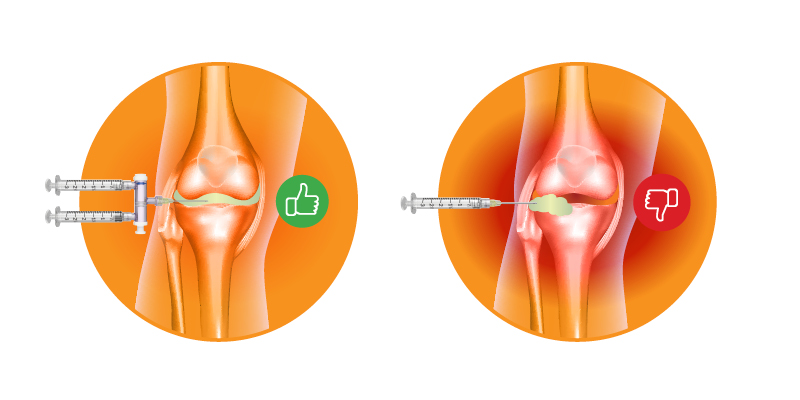
According to Anton Jarsky, Business Development Director at Elcam Medical, “They were running clinical trials with a drug injected into the knee, and they kept seeing the same thing – physicians had to aspirate, then manually exchange syringes to inject, while exposing the needle hub to open air.”
The technique itself wasn’t the issue; physicians were following established practice. The challenge appeared in the brief pause between steps. That moment when one syringe was removed and another introduced added uncertainty at the exact moment when precision and consistency mattered most.
The team realized that simplifying and streamlining the process could make a real difference. A device that allowed for a smoother transition between aspiration and injection could reduce complexity at the most delicate point in the procedure.
But while the idea was clear, bringing it to market required development, concept testing, human factor engineering, manufacturing scale, regulatory readiness, and quality-control systems. So they turned to Elcam (that manufactures close to 1MM flow control devices per day), and their patented prototypes were reborn as A-Tap™.
When Continuity Matters
Intra-articular joint injections, especially in the knee, are an important part of orthopedic care worldwide. In everyday practice and clinical research alike, clinicians rely on them, including intra-articular corticosteroids and Hyaluronic Acid, which the U.S. National Institutes of Health identifies as a mainstay of therapy for knee osteoarthritis.
The procedure usually involves two steps: joint aspiration (arthrocentesis) followed by delivery of medication into the same joint space.
Both steps depend on precise intra-articular needle placement to deliver medication accurately and keep the patient comfortable. Reaching that position takes skill and concentration, and clinicians most often rely on ultrasound guidance to help them place the needle correctly.
Beyond that point, however, aspiration, confirmation, and injection are often interrupted as the clinician disconnects the syringe, switches hands or syringes, and works to keep the needle steady before delivering the medication. That brief pause adds several challenges to the procedure:
- Needle movement can affect placement and increase patient discomfort
- Fluid leakage that adds unpleasantness for the patient and complicates handling at the site
- The break in focus can extend procedure time
Jarsky says there’s also a small but real risk of contamination while the system is open to air. “The infection risk is quite low, but if infection happens, it’s a big deal.”
In developing A-Tap™, Elcam aimed to answer the simple question: how can we preserve procedural flow at the most sensitive moment?
From Concept to an Elcam-Ready Product
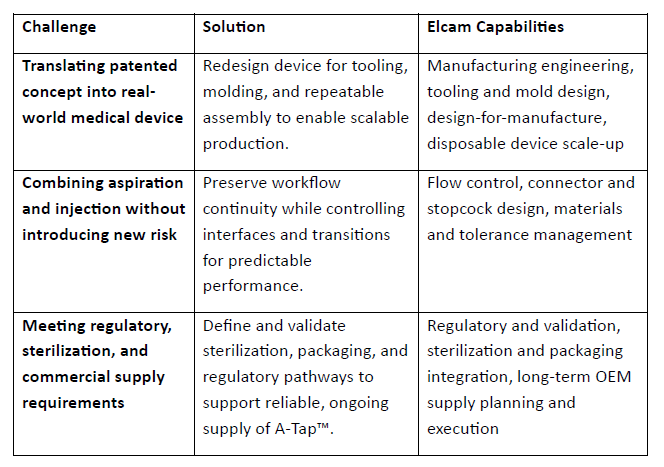
An approved patent and a viable concept, even with some clinical testing, don’t always translate into scalability, repeatability, or long-term support. A concept can function in limited settings and still fall short when it needs to scale, work reliably, and meet supply-chain demands.
To bring A-Tap™ to the finish line, Elcam took over product development, manufacturing scale, validation, and ongoing supply, turning the concept into a commercially available product. That work focused on:
- Evaluating design decisions for predictable handling and performance
- Refining tooling and molds to align with Elcam’s production standards
- Engineering the device to perform consistently across units, users, and clinical settings
- Integrating elements like packaging and sterilization into the finished product
At the heart of all this lay a single priority: making sure A-Tap™ checked the boxes that matter most to clinicians, simplifying the procedure in real-world use while supporting patient comfort.
Challenges In Creating This “Simple” Device
Turning a concept that looks simple in use into a device that performs consistently, meets regulatory expectations, and supports long-term supply introduces a unique set of challenges. With 50+ years’ experience in the medical device field, Elcam had the capabilities to meet these challenges.
Result: A Clinician-Ready Product
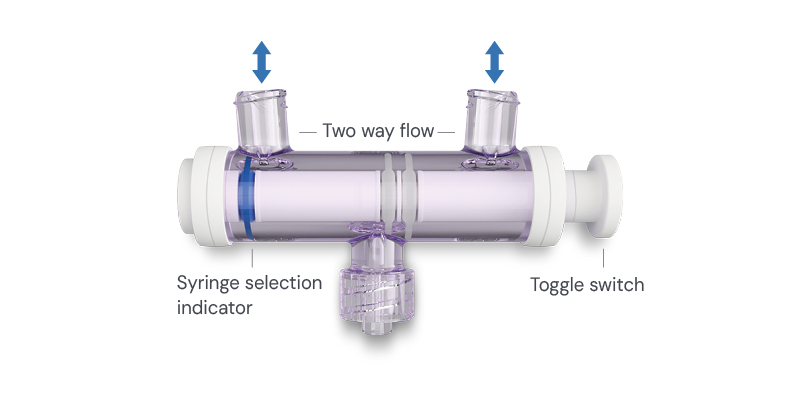
Elcam’s OEM experience across multiple clinical domains informed A-Tap™’s workflow-first approach. It supports established practice by removing disruption at a critical point in the procedure. Aspiration becomes a seamless verification step within a continuous workflow, and injection follows without breaking the sterile pathway or altering needle position.
For most clinicians, the value is apparent as soon as they see or handle the device. “Once orthopedists see the product, you don’t even have to explain, it’s immediately clear how it works and why it helps,” Jarsky says.
Clinicians who use A-Tap™ gain efficiency, control, and continuity during the procedure without additional equipment or added burden. They appreciate being able to focus on the joint rather than managing transitions between steps.
That continuity can also reduce procedure time. As Jarsky explains, “If you’re in a physician practice where you have to switch the syringes, it can take between a minute and a half to two and a half minutes. With this device, you can do it in almost under a minute. You spend less time with the needle inside the patient’s knee, there’s much less movement, and the procedure is cleaner overall.”
A-Tap™: An Iterative Advance That’s Changing The Game
Making A-Tap™ a reality relied on Elcam’s long-standing work with precision components, flow control, and sterile disposables, plus coordinated execution across manufacturing, validation, and quality systems. The focus throughout was practical: ensuring the device could perform consistently, meet regulatory expectations, and support real-world use at scale.
The result? A value-added workflow improvement that is easy to explain, demonstrate, and position within established product portfolios. A-Tap™ offers a simpler, safer, and more dependable way to perform a familiar procedure, making it straightforward for OEMs to integrate into competitive offerings.
Bringing A-Tap™ to market drew on Elcam’s decades of experience supporting commercial medical devices: navigating regulatory requirements, manufacturing constraints, and supply considerations that often determine whether a product succeeds.
Learn more how A-Tap™ makes a versatile and innovative addition to your product portfolio – https://www.elcam-medical.com/a-tap/