Providing high quality disposable flow control medical devices and solutions for the medical OEM industry, Elcam Medical is a world class producer of disposable medical devices and accessories for the OEM market, and a provider of innovative solutions for specialized flow control needs. Elcam Medical strives to provide the building blocks for a safer and more effective fluid management in medical areas such as Intensive Care, Anesthesia, Dialysis, Interventional Cardiology & Radiology and Oncology. We do so with a comprehensive understanding of the medical and clinical environment and through partnering with our OEM customers who are the medical industry’s leading companies.
As a worldwide leader of fundamental elements in fluid management systems such as stopcocks and manifolds in the OEM markets, Elcam maximizes efforts to provide qualitative customized solutions to our customers greatest needs and challenges. With a commitment to provide an excellent customer experience, Elcam Medical’s global teams incorporate into all our services, both professionalism and care in a way that exceeds industry standards.

Our Advantages – Numbers
0%
800 customers from
all across the globe
0%
Over 30% of the world
stopcock market share
0%
55 years of experience
and striving for excellence
Innovation and Continuous Development
Innovation and Continuous Development
Care for our customers and their success drives our innovation.
We innovate based on our customers’ needs in order to provide real solutions to real problems, and to offer a competitive advantage for our customers.
Elcam Medical develops, manufactures and sells innovative medical systems, devices and accessories for critical care applications. Our products promote several patient and caregiver safety issues, such as minimizing bloodstream infections, reducing the likelihood of tubing misconnections and disconnections and protecting healthcare workers from exposure to infectious diseases or hazardous drugs.

Quality Assurance
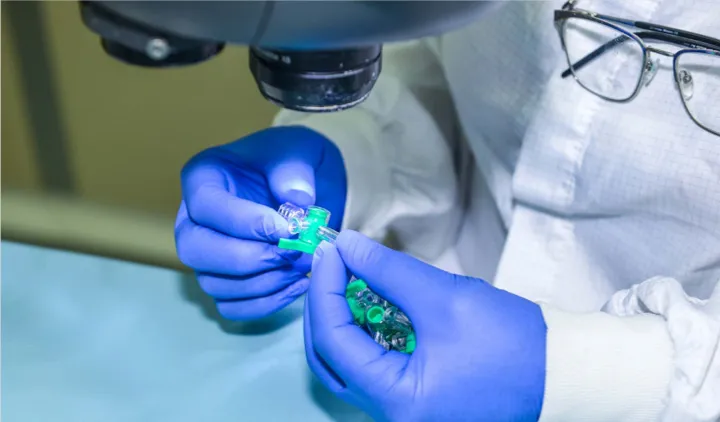
Quality Assurance
Elcam Medical is experienced in working under tight deadlines, without compromising quality. Elcam is committed to working according to all applicable laws and regulations, and operates in compliance with 21 CFR 820 and ISO 13485:2016 throughout the product life cycle. This includes R&D activities, production, sales, marketing, and post market surveillance, for continuous improvement in product quality, product safety, effectiveness and customer service. Elcam acts to maintain the effectiveness of its quality management system.
Customer Experience
Your optimal OEM partner
Customer Experience
Your optimal OEM partner
Elcam is a medical OEM industry leader and expert in the medical device markets. Elcam believes that the small details make a difference and strives to provide the highest quality components and the support needed for building the best final devices.
Elcam expertise lays not only in producing products, but also in providing engineering and regulatory support across the product’s life cycle, from design and development, through manufacturing and registration, up to product launch and post marketing.
Elcam believes in building and nurturing long term, stable relationships with our customers and partners, sharing risks, knowledge, and expertise, so we can grow and prosper together.

Seamless Integration

Seamless Integration
Elcam recognizes that our solutions are only a small part of our customers’ final products. Therefore, we believe that the Elcam products need to mesh seamlessly with your finished product.
Elcam’s exceptional customer experience and supporting services ensure that our devices and components are easily integrated into the customer final products and sets:
- Product customization
- Engineering support
- Validation & Verification (V&V) support – mutual V&V integrated plans, V&V reports
- Regulatory support – Biocompatibility testing, Regulatory registration (CE, 510(k))
- Customer complaint handling and QA customer support
- Global presence with strong customer support units for daily services
- Assistance with MarCom content preparations
Joint Validation & Verification
Joint Validation & Verification
A faster and smarter way to the market.
Over the past few years, it has become evident that medical device companies are looking for ways to expedite approval processes of new designs. The development of a new customized service, a highly effective process for Joint Validation & Verification activities is a service offered by Elcam in order to meet this need and remove the burden from the medical device provider. The process complies with ISO 13845 standard requirements.
“Employing Elcam’s Validation & Verification (V&V) testing services not only helped the project move along more expediently, but it allowed our internal resources to be utilized more efficiently. Elcam was professional and comprehensive with all the required testing. We will certainly take advantage of Elcam’s V & V services for future partnership opportunities”.
.Braun Melsungen
R&D Manager

Product Entirety
Vast Product Range
Product Entirety
Vast Product Range
Elcam offers the widest range and variety of high quality disposable medical devices for fluid control suitable for your everyday or critical needs.
Supply management has always been a vital aspect of every OEM company activity, a challenge that becomes more complex with the growth of any company. Elcam’s expanded portfolio found in our various divisions – Elcam Devices, Elcam Stopcocks, Elcam Accessories, and those created through our custom design services constitute a comprehensive solution to support our customers.
Elcam’s OEM leadership position in medical fluid control enables your company to source all your components from only one supplier. Elcam offers a complete package with components needed to create a wide variety of sets for IV, monitoring, dialysis, nutrition and other applications:
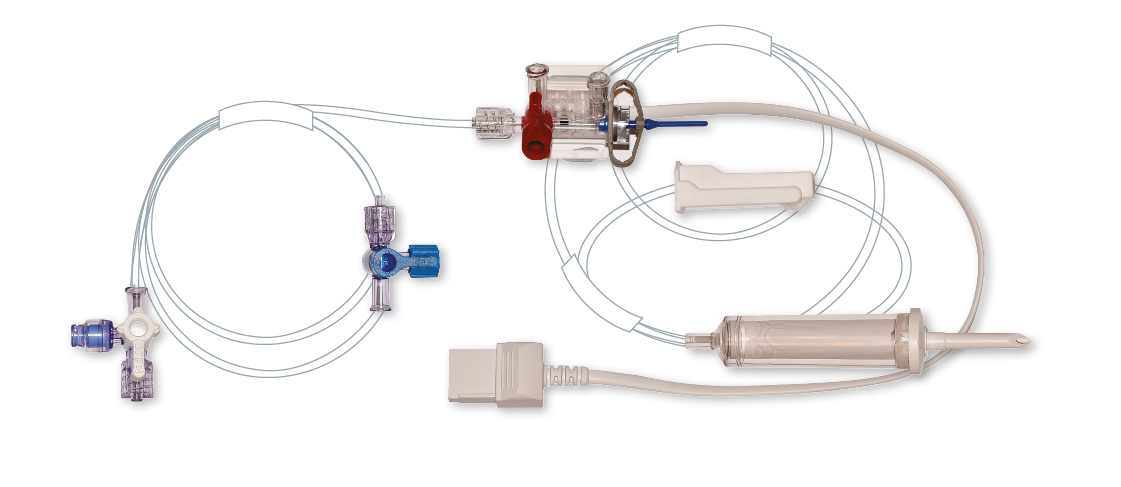
Customizations and Tailor-Made Adjustments

Customizations and Tailor-Made Adjustments
Our products can be customized according to your specific needs.
Elcam Medical offers its expertise, world class equipment, and production capacity for custom made products for you. Our customization capabilities are broad and include unmatched configurations, flow rates, handle styles, and variety of high performance materials.
Elcam Medical is committed to understanding your unique requirements, applying our 40 years of experience and advanced manufacturing capabilities to every project, in order to design, develop and manufacture comprehensive solutions for your customers need.
Our renowned custom designed stopcocks, from specialty stopcocks for laparoscopy and manifolds for oxygenators, to our integrated pressure transducers, constitute just a few examples of Elcam’s capabilities to provide tailor made adjustments.
Our customization capabilities and vast product range have made Elcam Medical a preferred supplier for many of the leading medical device manufacturers around the world.
Striving for flawless medical devices
Striving for flawless medical devices
Having zero errors in treating patients is the overriding objective for quality in the medical device and healthcare industries. Elcam understands the importance of providing our customers with high quality products and constantly strives to build more robust products and processes, aiming to insure product performance as well as the products’ safety and effectiveness.
Our robust QA and Regulatory system grants confidence in distributing safe and effective devices that meet the regulatory requirements and strictest standards of the medical device industry. Elcam operates in compliance with 21 CFR part 820 and ISO 13485:2016 throughout the products life cycle. Elcam holds CE Mark for the European market and many of our products marketed in the US are 510(k) cleared devices. In addition, we support our customers’ regulatory submissions in their respected territories, including US, Europe and Asia.
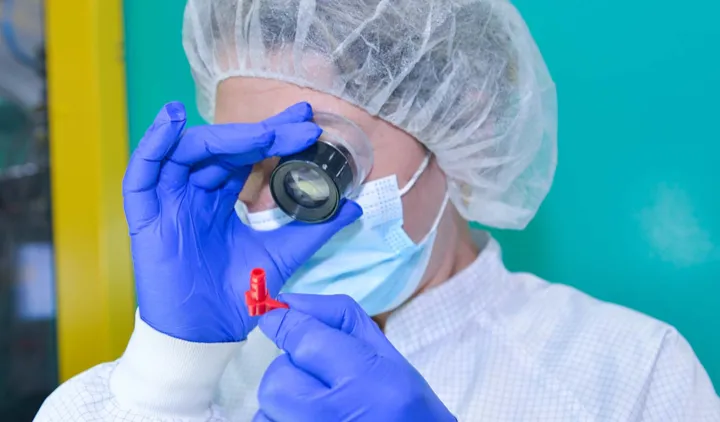
Clinical Expertise
Cooperation with the industry

Clinical Expertise
Cooperation with the industry
Clinicians, healthcare professionals, as well as clinical specialists of medical device companies, best understand the needs and requirements for safe and effective medical procedures.
Elcam’s close cooperation with OEM customers, key clinicians and inventors to explore the medical market on an ongoing basis keeps us familiar and committed to the medical market needs, trends, and benchmarks.
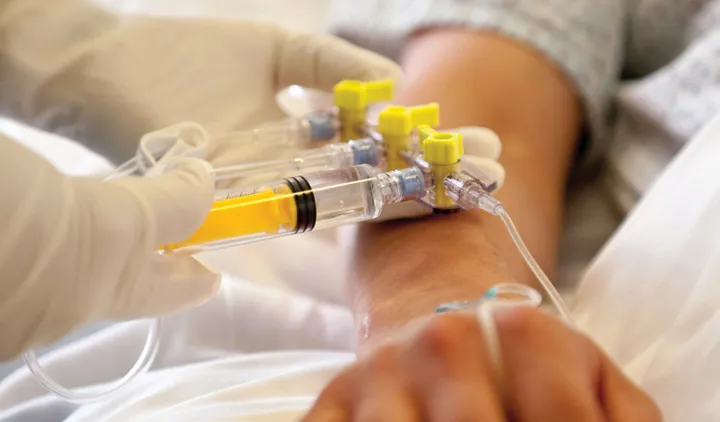
Safety is in the Details
Safety is in the Details
We strive to maximize safety through focusing on improvements of small yet significant needs.
Elcam is leading the effort to improve safety in medical fluid management in acute settings, such as pressure monitoring, IV therapy, drug delivery and dialysis.

Safety is in the Details
We strive to maximize safety through focusing on improvements of small yet significant needs.
Elcam is leading the effort to improve safety in medical fluid management in acute settings, such as pressure monitoring, IV therapy, drug delivery and dialysis.
Advisory Board
Elcam Medical employs Israeli and American advisory boards that meet regularly every year.