Elcam is continuously working to enhance both our product offerings and support services. In this edition of our newsletter, we highlight Elcam’s Oncology portfolio and capabilities. You’ll also find updates on our recent accreditations from MedAccred, EcoVadis, and our ESG report, as well as introductions to a new team member
Oncology
In recent years, awareness has increased about the harmful effects that cancer-fighting drugs can have on healthy organs. These risks impact not only patients but also the medical staff who handle these medications daily. To address this challenge, new solutions have been developed to protect both patients and caregivers by eliminating exposure to hazardous drugs throughout the entire preparation and treatment process.
Following are a few examples of Elcam’s products for Oncology applications
Explore our Oncology product line
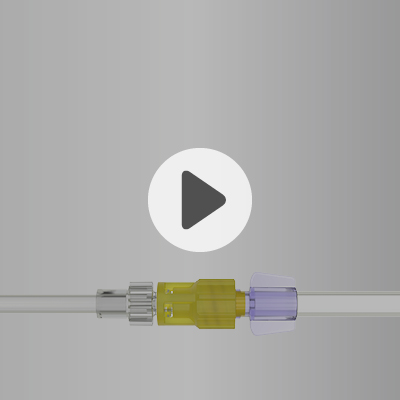
OLAV™
OLAV™ is a closed male Luer connector designed to minimize exposure to hazardous drugs by creating a closed system for syringes and IV sets across preparation, transport, administration, and disposal. Its normally closed valve opens only when connected and automatically seals upon disconnection to prevent leaks or residues. A secure locking collar ensures a permanent, leak-free connection, reducing the risk of accidental drug spillage.
Key Advantages of Elcam’s OLAV:
- Meets NIOSH requirements for safe handling of hazardous drugs
- Automatic closure prevents spills and drips
- Secure locking collar avoids accidental disconnection
- Needle-free, swabbable male tip
- Microbial barrier on male luer lock
- Ergonomic grip with disconnection indicator
- Fits standard female luer connections
- Made from durable polysulfone and LSR
- Minimal changes to existing practice required

Safe2 Rotator™
Secure, reliable connections of medical tubing are essential for safe and effective IV therapy. Leaks or accidental disconnections can cause serious harm to patients, and in Oncology, where hazardous drug therapy is involved, the stakes are even higher due to the risks posed to healthcare teams from drug exposure.
Elcam’s Safe2 Rotator™ (S2R) is a unique polyethylene flexible connector designed to enhance safety for both patients and medical staff. By reducing mechanical stress during Luer lock connections, it helps prevent cracks, leaks, and accidental disconnections, helping to ensure safer and more reliable IV therapy.
Learn more about S2RMedAccred
We are excited to share that Elcam has expanded its MedAccred accreditation, first achieved in 2022 for Plastics Injection Molding, to now include Assembly processes in our Israeli facilities. Elcam is proud to be the first company in Israel to earn this recognition, awarded after meeting the rigorous audit requirements of MedAccred subscribing members, including Baxter, BD, Boston Scientific, Edwards Lifesciences, Johnson & Johnson, Medtronic, Philips, Roche Diagnostics, and Stryker. Together, these accreditations highlight our commitment to delivering the highest quality and most reliable medical devices, always with patient safety at the forefront.
Learn about our quality standards
Ecovadis
Elcam is proud to announce that we have been awarded a Silver Medal in the 2025 EcoVadis sustainability rating, ranking us among the top 15% of companies in our industry. This recognition reflects our ongoing commitment to reducing environmental impact, fostering positive social change, and advancing our sustainability journey. We remain dedicated to making a meaningful difference for people and the planet.
View our sustainability initiatives
2024 ESG Report
Elcam is pleased to present the 2024 Environmental, Social & Governance (ESG) report highlights and updates, showcasing our ongoing commitment to sustainability, equity, and corporate responsibility.
Environmental Achievements:
- 2.23 million tons of CO₂ emissions avoided
- 97.5% improvement in energy efficiency (COP) over 5 years
- 13% of total energy sourced from our own renewable systems
Diversity & Inclusion:
- Team members from 33 countries
- 22% of managers in Israel from underrepresented minority groups
- 46.5% of our workforce are women

New Team
Welcome Einav Segev – Product Manager, Patient Monitoring & Interventional Procedures
Einav joined Elcam Medical in June 2023 as an R&D Project Manager and, in June 2025, became part of the Marketing and Business Development team.
Einav holds a BSc in Nutritional Sciences from Tel-Hai College and an MBA in Life Sciences Management from the Technion – Israel Institute of Technology. She brings extensive experience in leading cross-functional teams and driving operational excellence, with a background in management consulting, client engagement, and team leadership.
At Elcam, Einav oversees the full development lifecycle of patient monitoring and interventional procedure devices—from concept through verification, validation, and launch, managing timelines, budgets, risk, and regulatory compliance, all in alignment with our internal quality systems.
Einav shared: “I’m proud to be part of a professional, safety-driven, and innovative team, and to help bring reliable products to market for clinicians and patients.”
Contact Einav

Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
Medtec China
24 – 26 September
Shanghai, China
Booth #2D001
Medical Technology Ireland
24-25 September
Galway, Ireland
Booth #L48
Compamed
17-20 November
Duesseldorf, Germany
Booth #8aE28