Minimize exposure to hazardous drugs with OLAV™
Elcam’s closed maler Luer connector creates a closed system for syringes and IV administration sets, minimizing exposure during drug preparation, transport, administration and disposal
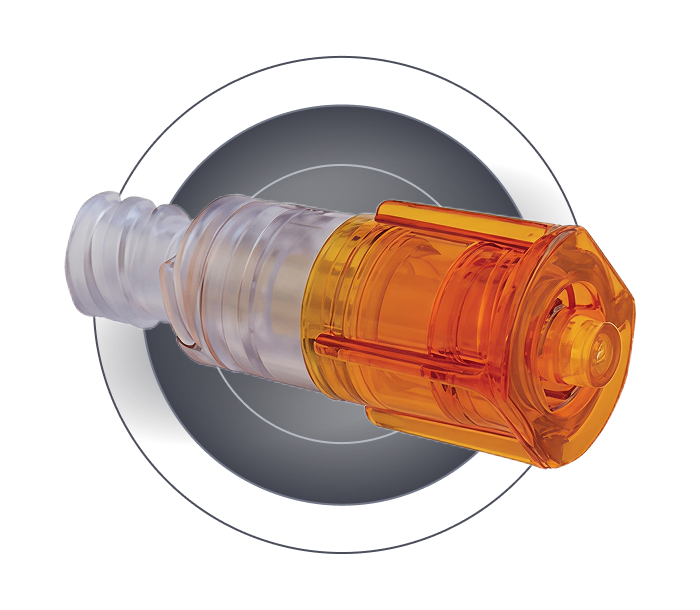
Female Luer Lock
Non-detachable female Luer Lock
Grip area
Closed male Luer Lock
Everything you need to know about the product
OLAV™ is a Closed Male Luer Connector. Its primary purpose is to minimize dripping of liquids and drugs from a male luer connector, particularly in the context of hazardous drug administration. It is designed to reduce exposure to hazardous drugs during the entire process, including drug preparation, transportation, administration, and disposal, by creating a closed system for syringes and IV administration sets.
OLAV™ minimizes exposure to hazardous drugs by creating a closed system for syringes and IV administration sets across all stages: preparation, transportation, administration, and disposal.
This is achieved through several key design and functional mechanisms:
• Automatic Closure: The OLAV features a normally closed male luer valve that opens only when firmly connected to a female luer for infusion and automatically closes upon disconnection. This design actively prevents leakage of fluid or medication, thereby minimizing spills, drips, and dribbles. The male luer tip is also level with the valve mechanism, ensuring minimal drug residues remain on the tip after disconnection.
• Secure, Permanent Connection: Its female luer connector includes a non-detachable locking collar or ratchet mechanism. This design ensures a permanent connection to the syringe or the end of a secondary IV administration set, significantly reducing the risk of hazardous drug spillage due to inadvertent detachment or accidental disconnection.
Minimizing exposure to hazardous drugs, particularly antineoplastic (chemotherapy) drugs, is critically important due to their inherent toxicity. These drugs are not only toxic to cancerous tissue but also to healthy organs.
Long-term contact with such drugs has been shown to have mutagenic and even cancer-promoting effects. Therefore, devices like OLAV are developed to enhance healthcare staff safety in handling these drugs and to minimize this exposure.
The compatibility of OLAV™ was demonstrated through a study conducted by Elcam Medical with a representative selection of hazardous drugs, chosen in accordance with NIOSH alert and American Cancer Society guidance. These drugs were selected from known cancer treatment family groups based on their mechanism of operation, including Anti-metabolites (Fluorouracil/5-FU), Alkylating Agents (Cisplatin, Cyclophosphamide) Anti-tumor antibiotics (Doxorubicin/Adriamycin), Mitotic inhibitors (Paclitaxol/Taxol), Topoisomerase inhibitors (Etoposide), and Immunotherapy (Bevacizumab/Avastin), as well as Intralipid 20% (known to be incompatible with many amorphous plastic materials).
The tests showed that OLAV maintains its performance and integrity when exposed to these hazardous drugs.
OLAV™ is designed to minimize microbial ingress into the drug administration system. Its male luer lock is specifically designed with a microbial ingress barrier. The effectiveness of OLAV™ in preventing bacterial ingress was demonstrated in a third-party study. In this study, the device was challenged with high concentrations of four common nosocomial infection microorganisms: Staphylococcus aureus, MRSA, Enterobacter aerogenes, and Pseudomonas aeruginosa. The results showed bacterial counts of less than 1 CFU/2ml (<1 CFU/2ml) in each test group, proving that O-LAV™ can serve as an effective microbial barrier. Additionally, its automatic closure upon disconnection also contributes to reducing microbial contamination.
When OLAV™ disconnects from a female luer connector, its internal valve closes automatically, which prevents the leakage of fluid or medication. This automatic closure design is intended to reduce drug exposure and microbial contamination, and to minimize spills, drips, and dribbles. The male luer tip is positioned level with the valve mechanism, ensuring minimal drug residues remain on the tip.
Can’t find the answer you’re looking for? Please chat to our friendly team.
Contact Us
This page seems also relevant to my colleague