OLAV™ Closed Male Luer Connector: Addressing Hazardous Drug Exposure in Healthcare Settings
Einav Segev
The Scope of Hazardous Drug Exposure
Healthcare workers handling hazardous drugs (HD) in oncology settings face significant occupational exposure risks. According to ONC Nursing News, “The Centers for Disease Control and Prevention estimates that more than 12 billion doses of HDs are handled annually by health care workers, especially pharmacy and nursing staff. In the United States alone, over 8 million health care workers a year are exposed to HDs. These startling numbers, which will only increase as the population ages, should motivate all oncology nurses to stay informed regarding HD safety.”
The challenge extends globally. The Parliament Magazine reports that “Every year more than 12.7 million health professionals in Europe, including 7.3 million nurses, are exposed to carcinogenic, mutagenic and reprotoxic hazardous drugs.”
The CDC notes that “About 8 million U.S. healthcare workers are potentially exposed to hazardous drugs. Inhalation and skin contact or absorption are the most likely routes of exposure.”
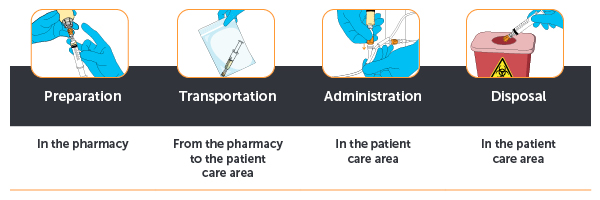
In response to this pressing need, the O-LAV™ Closed Male Luer connector plays an important role in minimizing hazardous drug exposure throughout all phases of hazardous drug handling: during preparation, transportation, administration, and disposal.
How OLAV™ works
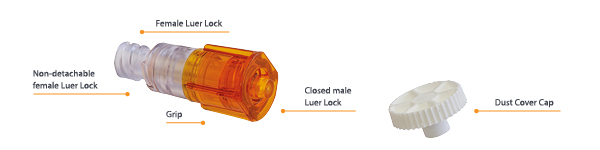
The OLAV™ Closed Male Luer Connector is a valve designed to minimize dripping of liquids and drugs from syringes and IV administration sets during hazardous drug administration. It is comprised of a normally closed male luer valve at the proximal end that opens when attached to a female luer or needlefree connector to allow infusion. On disconnection, the valve closes automatically, minimizing leakage of fluid or medication. The male luer tip is level with the valve mechanism, ensuring minimal drug residues remain on the tip.
The device includes a female luer connector at its distal end, allowing connection to medical devices for fluid administration such as syringes or infusion sets. The female luer connector is designed with a ratchet mechanism (non-detachable locking collar), preventing the risk of hazardous drug spillage following accidental detachment of the OLAV™ from the syringe or infusion set.
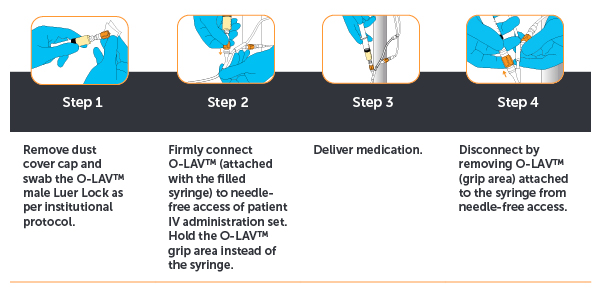
Ease of Use During Administration
Using OLAV™ is safe and simple comprising four easy steps.
Hazardous Drug Compatibility
Hazardous drugs are incompatible with many polymers used to manufacture disposable medical devices for drug administration, which may lead to the degradation of the polymer material, leading to crazes (stress marks), cracks, breakages and ultimately to device dysfunction.
The compatibility of the OLAV™ device with hazardous drugs was demonstrated in a study performed by Elcam Medical in cooperation with Western Galilee Medical Center’s pharmacy. The device was exposed to hazardous drugs including cisplatin, cyclophosphamide, fluorouracil, doxorubicin, paclitaxel, etoposide, and bevacizumab. Drug selection was based on frequency of use, toxicity to medical staff, and ability to attack OLAV™ flow path raw materials (Polysulfone).
Samples of OLAV™ were aged to simulate 5 years shelf life, sterilized, and exposed to these drugs for 24 and 96 hours. The exposed components were tested for leakages according to ISO 80369-7 standard and for flow rate. All tested components and groups passed the tests, and products performed according to their specifications. No degradation in product performance was observed after either 24 or 96 hours of exposure.
Key Advantages
OLAV™ is designed for both safety and ease of use:
- Compatible with NIOSH requirements for safe handling of hazardous drugs
- Automatic closure upon disconnection — minimizes spills, drips, and dribbles
- Non-detachable locking collar — prevents accidental disconnection from syringe or IV set
- Needle-free, swabbable male tip
- Male luer lock designed with microbial ingress barrier
- Ergonomic grip area that alerts the clinician to disconnection position
- Compatible with standard female luer connections
- Manufactured from polysulfone and LSR
- Requires minimal changes to current practice
Used in oncology applications worldwide, the OLAV™ Closed Male Luer Connector effectively minimizes spillage of liquids and drugs through its automatic closure mechanism and closed system design throughout all phases of hazardous drug handling.
For more information about the OLAV™ Closed Male Luer Connector and its application in hazardous drug safety protocols, visit our product information page.
Special thanks to Anton Jarsky for his collaboration and significant contributions to the writing of this article.