Elcam continuously strives to improve product offerings and supporting services. This edition of our newsletter highlights Elcam’s tube assembly capabilities including the Marvelous™ with tube and spikes with tube. We share the results of our annual customer satisfaction survey, update on the upcoming EU MDR regulation, and introduce our new Chief Technology Officer.
Tube assembly Capabilities
As part of Elcam’s continuous efforts to expand product range to best answer stopcocks needs, Elcam is offering its stopcocks with a variety of extension tube lines as cost effective sub-assemblies.
Elcam offers the widest variety of tube fitments for bonding, or oversizing, off the shelf or tailor made and also offers its professional support in building and tweaking your bonding process.
Following are some great examples of Elcam’s tube assembly capabilities and offerings
Learn more about reducing plasticizer-induced stress cracking
Marvelous™ with tube
Optimize inventory management and enhance safety with the Marvelous™ stopcock and extension tube. The innovative design of Marvelous minimizes contamination risks and streamlines medication delivery. The inner channel and responsive valve provide superior protection against contamination, air entry, and residual medication. The extension tube offers greater flexibility and easy access without frequent disconnections further minimizing infection risks.
Learn more about the Marvelous
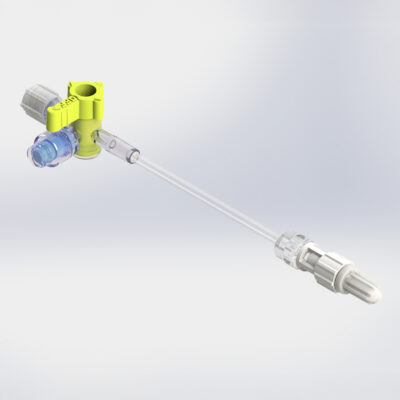
Stopcock with tube
Elcam’s Stopcock with extension Tube line offers ultimate convenience and efficiency while maintaining the top-quality standards customers have come to expect. Elcam’s wide range of stopcocks and tubes allows you to customize your setup to fit your specific needs.
Explore our stopcocks with tubes varietySpike With Tube
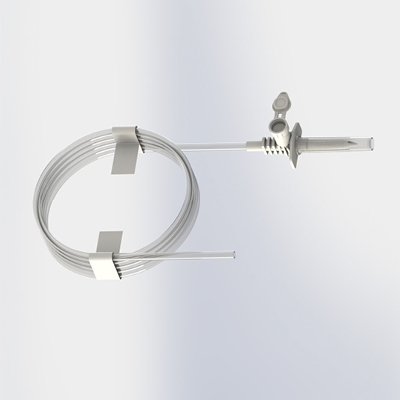
Elcam is expanding its tube assembly capabilities to include Spikes with tubes which can seamlessly integrate with existing IV setups. Elcam’s spikes are designed to meet a variety of applications including IV, drug transfer and chemotherapy. The extension tube provides greater flexibility, allowing easy access as well as reducing inventory management costs while maintaining the highest quality standards.
Learm more about our spikes
Customer satisfaction survey
Elcam recently completed the analysis of its annual customer satisfaction survey, which was sent out in December. We extend our gratitude to all who took the time to provide valuable feedback, which supports Elcam’s continuous efforts to enhance our services.
Amir Bohadana, Elcam’s Chief Sales Officer concluded: “We were happy to see that the efforts we have invested during 2024 to uphold our promise of consistent delivery and support have resulted in continued customers satisfaction in responsiveness time, on-time delivery, quality and technical support. We are aware there are still areas where we need to improve as we observed a decrease in NPS score, which reflects the customers loyalty levels and aim to improve the score in the upcoming years”.

EU Regulation 2023-607 MDR News
As you may be aware, the European Regulatory Agency ECHA has set a deadline for the use of DOTE in products distributed in the European Market. This is a forced change that applies to all operators involved in the supply chain of plastic components, from suppliers of chemicals (substances) to manufacturers of products containing such chemicals.
The deadline for discontinuing the use of DOTE is May 1, 2025. After this date, Elcam will no longer be allowed to purchase raw materials, produce, or supply products containing DOTE. The new rule prohibits the use of DOTE even at a concentration below 0.1% w/w. All raw material suppliers have informed us of their decision to comply with the deadline and phase out all products containing the DOTE stabilizer.
To mitigate the impact of this discontinuation, Elcam has already replaced affected part numbers with new, fully compliant alternatives. These alternatives meet the same or improved quality and performance standards and are available for sampling upon demand. Our Customer Service team has already provided a comparison table of old and new part numbers to ensure a smooth transition. Elcam will be happy to support you in this transition and with any documentary requests.
Contact our customer service for more details
Team News
Elcam is delighted to announce the appointment of Matan Gedulter as our new Chief Technology Officer. Matan holds a Bachelor of Science in Biomedical Engineering and a Master of Business Administration, bringing 20 years of experience in medical device R&D. Over the course of his career, he has driven innovation in developing devices across multiple clinical fields and is the inventor of numerous patents in the industry.
Matan shared his excitement about joining Elcam, stating: ‘I am enthusiastic to join Elcam’s professional, experienced leadership team and to have the opportunity to make an impact on the medical device industry on a global scale. At Elcam, I will focus on advancing our portfolio of high-quality, state-of-the-art medical devices and components, reinforcing Elcam’s commitment to delivering trusted solutions that enhance patient care worldwide, and tailoring customized, optimized solutions to increase our customers’ value proposition.”
Join us in welcoming Matan to the Elcam family
Contact Matan

Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
Medtec Japan
9 – 11 April
Tokyo, Japan
Booth 1503
