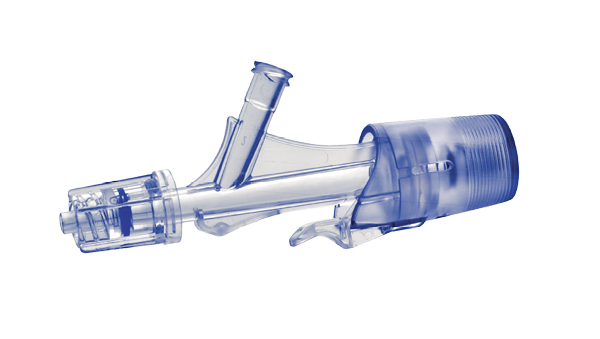
Y-Click™: New Approach to Hemostatic Valve Technology for PTCA Procedures
Tomer Gil
Hemostatic Valves in Interventional Cardiology
Hemostatic valves are crucial components in medical devices used for catheter-based procedures. These valves serve a vital function by preventing bleeding during interventions and maintaining a clean surgical field, while allowing medical instruments to pass through and maintain a sealed environment. In minimally invasive procedures, particularly Percutaneous Transluminal Coronary Angioplasty (PTCA), hemostatic valves are essential for ensuring procedural success and patient safety.
The Critical Role of Hemostatic Valves in PTCA
PTCA is a minimally invasive procedure designed to open blocked coronary blood vessels and improve blood flow to the heart muscle. During this procedure, physicians insert a catheter, guiding it to the coronary arteries. After injecting dye for X-ray visualization, they use a balloon catheter to compress blockages against the artery wall, often placing a stent to keep the artery open.
Throughout this complex process, hemostatic valves play an indispensable role by preventing blood loss during instrument manipulation, maintaining a sealed environment for the procedure, enabling smooth introduction and withdrawal of various devices, and contributing to overall procedural efficiency and patient outcomes.
Y-Click Hemostatic Valve Design
Elcam Medical’s Y-Click represents a significant advancement in hemostatic valve technology for PTCA applications. Developed in close collaboration with interventional experts, this easy-to-use hemostatic device is suitable for both diagnostic and interventional procedures in cardiology.
How Y-Click Transforms the PTCA Experience
The ergonomically designed Y-Click provides easy maneuvering and may help to reduce blood loss during the introduction, use, and withdrawal of diagnostic and interventional devices used in angioplasty procedures.
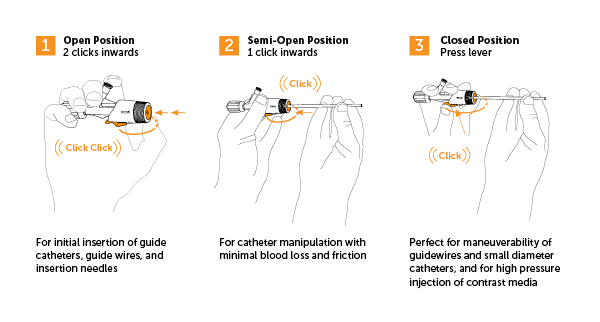
What sets it apart is its unique three-stage lever-operable valve with distinct positions:
3 Key Differentiators That Benefit Cardiologist
- One-Handed Operation for Enhanced Procedural Control
Y-Click’s design enables one-handed operation, freeing the cardiologist’s other hand for additional tasks or instrument manipulation. This single-hand functionality represents a significant advantage in the busy interventional setting, allowing for more efficient procedural workflow and potentially reducing overall procedure time. - Intuitive Audible Click System
One of Y-Click’s standout features is its audible “clicks” that clearly indicate position changes. The “click” provides immediate feedback to the surgeon without requiring visual confirmation, enhancing confidence and precision during critical moments of the procedure. - Blood Loss Prevention
Y-Click effectively prevents back bleeding in the Closed position and minimizes back bleeding in the Semi-Open position, which balances instrument maneuverability with minimal bleeding. Exclusive to Y-Click, this middle position allows for catheter manipulation with reduced friction while still maintaining an effective seal. Additionally, Y-Click offers a high inner diameter that accommodates a wider range of instruments while maintaining excellent sealing properties.
Advanced Safety Features
Beyond its primary benefits, Y-Click incorporates several advanced safety mechanisms. It prevents air ingress into the device when the valve is in Semi-Open and Closed positions. The self-closing safety mechanism automatically moves from Semi-Open to Closed position when high pressure is applied during contrast media injection, and it provides good signal keeping in both Closed and Semi-Open positions.
Technical Performance That Meets Demanding Needs
The Y-Click is engineered to perform in demanding interventional environments:
- Compatible with automatic contrast media injectors (up to 15cc per second and 600 psi)
- Suitable for use with 9 Fr or smaller guide catheters
- Works with 0.014″ – 0.038″ diameter guide wires
- Meets strict biocompatibility requirements (ISO 10993-1)
- FDA 510(k) cleared (K060759)
Y-Click’s Impact on PTCA Procedures
The Y-Click hemostatic valve represents a significant advancement in PTCA technology. By focusing on surgeon-centric design with one-handed operation, audible position confirmation, and superior blood loss prevention, Y-Click enhances procedural efficiency while potentially improving patient outcomes.
For interventional cardiologists seeking to optimize their PTCA procedures, Y-Click offers a combination of intuitive operation, enhanced control, and advanced safety features not found in conventional hemostatic valves.
You may also be interested in:
https://www.elcam-medical.com/wp-content/uploads/2024/03/Y-Click_REV7A_03-2024_v1.pdf
Elcam’s new manufacturing facility in the Dominican Republic is progressing as planned. We anticipate completing the building envelope by the end of the year, with the majority of exterior walls finalized by May 2025.
The next phase of construction will focus on the interior, including cleanrooms, assembly and injection areas, a logistics center, and offices. Elcam is aiming for the facility to be fully operational by September 2026.
This strategic expansion underscores Elcam’s dedication to improving service for its North American clients and strengthening supply chain reliability. Additionally, the new facility is expected to create 160 new jobs within the local community.
Click to see our progress
Elcam is proud to announce the upcoming establishment of a new manufacturing plant in the Dominican Republic at a projected investment of $80 million.
The planned manufacturing facility is expected to start operations in 2026. It will include assembly and injection departments, a logistic center and local offices. The new facility is expected to generate about 160 jobs in its first stage of operation.
This expansion is an important layer of Elcam’s strategy plan. The new facility will enhance our capability to serve our clients in North America and further ensure supply reliability.
Through this webpage, we will keep you updated on important developments in the process
For further details you can contact our team through our website contact page
At Elcam, we believe that sustainability is a way of life. Expanding our commitment to renewable energy, we installed solar photovoltaic panels on the rooftops of Elcam Italy. This project is part of our efforts to significantly reduce our environmental footprint through energy-efficient practices,
.1,627 panels covering 3,100m2 —
- Produces 650kWp of electricity
- Reduces costs by 30%
- Cuts CO2 emissions by 30%
This impressive project, which was funded by EU funds from the Emilia-Romagna region, complements Elcam Israel’s solar panel project.
Together, these sites span 2.5 acres of photovoltaic panels, generate 1,500,000 kWh of electricity annually, and have achieved 1.6 million kilograms of CO2 emission savings since their installation.
Through this and other environmentally responsible actions, Elcam Medical continues to demonstrate leadership in sustainability and energy management within the industry.
Watch our video
Joint Verification and Validation (V&V)
Michael Segev I 19.03.2023
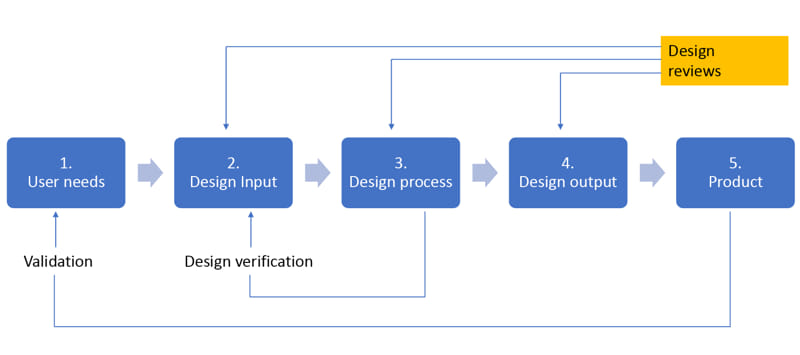
Design Verification and Validation (V&V) are independent procedures that are utilized in conjunction to ensure that a product meets its specification requirements and fulfills its intended purpose.
Design Verification ensures that a product meets the design specification. In the development phase, verification procedures include performing special tests, models, analysis, or simulating product performances.
Design Validation ensures that the product meets the intended use in the field (Hospitals, Medical centers, etc.). Design Validation involves human factor engineering studies, clinical experiments, or literature review.
Essential steps in product development process for V&V:
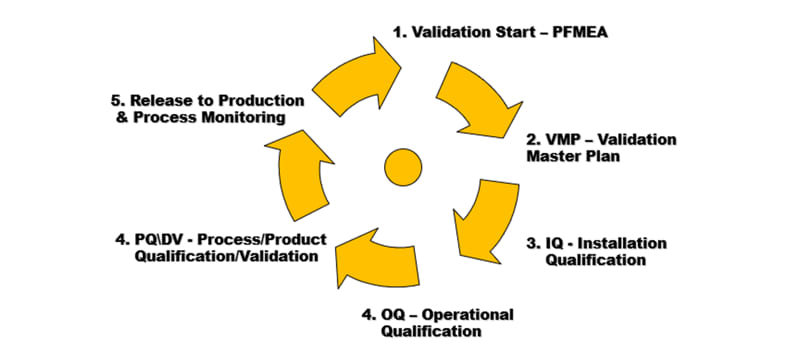
Process Validation demonstrates with a high degree of assurance that the process can create products that can be consistently manufactured while meeting predetermined specifications within stated parameters. Process Validation will include IQ, OQ, and PQ to qualify the production process.
Design V&V and Process Validation stage, as part of the Product development stage
As an OEM manufacturer, Elcam performs the Design Verification and Process Validation. Elcam carries out Design Validation when developing a new product that involves a change in the intended use. This process includes coordinating with the customer, depending on whether the new product is a combination product, part of a customer’s product, or the final product.
Joint V&V Program
Verification and validation during the implementation or transfer of components and products from development to production can be particularly challenging in terms of time and effort, especially for companies dealing with resource constraints, meeting regulatory requirements and maintaining their core capabilities.
A mutual V&V Integration program that includes inputs and cooperation of both the component manufacturer and the medical device company, will alleviate the workload of the medical device provider. This program can significantly accelerate the approval process and time to market of the end product, in addition to saving significant resources for the medical provider.
It is believed, backed by extensive experience and evidence, that adopting a collaborative model of mutual V&V for introducing a new component (e.g., stopcock, luer connector, drip chamber) into a medical device or medical set, will result in better process efficiency, eventually translating into cost and time savings for both sides. This will facilitate collaboration which creates synergic value, minimizes variability in the process, and meets critical quality requirements with the least amount of time, resources, and costs used.
The Joint V&V service program provided by Elcam serves to streamline the V&V process of integrating a new component into a medical device or a set. This service focuses on building a single mutual V&V plan instead of two separate plans to utilize resources most efficiently.
Joint V&V Program includes the following package, or partial package, as required specifically in each case:
- CRD/MRD (Customer Requirement Document/ Marketing requirement Document)
- Product specification accordingly
- DFMEA, UFMEA
- PDR, DDR’s and CDR
- Verification test report
- Shipment test report, Biocompatibility test report, Sterilization validation, and other documents required for CE, MDR, or FDA submission
- Production line design, process flow chart
- PFMEA, VMP (Validation Master Plan)
- IQ, OQ and PQ reports
- DHFs and DMRs and additional information involved with the customers’ needs
In conclusion, Elcam’s joint V&V service is a uniquely managed V&V process that enables the medical device manufacturer to save crucial time and money when approving a new product or replacing medical components in combination with customers’ products.
In addition, utilizing this service can aid in obtaining regulatory approval for the product. Therefore, it is advisable to consider utilizing and enhancing the V&V service when selecting components and suppliers. The value you get is the peace of mind that comes from having greater transparency and assurance in the V&V processes and quality standards.
Environmental, Social, and Governance Responsibility
Hadas Bibi Eizenberg I 17.08.2022
Environmental, Social and Governance (ESG) responsibility is a framework by which organizations demonstrate integrity regarding social, environmental, ethical, and economic issues. It is a way to take action to support the vision, mission, and values of an organization, rather than relegating it solely to a written statement.
ESG is not a side issue, or a hassle to be quickly disposed of. Rather, it is a strategic approach which suggests that organizations voluntarily include social, environmental, and ethical considerations in their decision-making process in order to ensure long-term success.
ESG is constantly growing and evolving as an increasing number of countries as well as global and local organizations decide to take action to ensure a better world. There is increasing recognition that in order to have a good life, we all need to take care of one other, especially as the pressure on limited environmental resources becomes increasingly challenging. Since we have only one planet, our actions today impact us all tomorrow.
Elcam Medical is committed to ESG responsibility. We believe it is not a goal, but a long-term journey where improvement comes by diligence and perseverance; it is the way that our organization operates in the world and as a way of life.
In 2022, Elcam Medical received a silver medal from EcoVadis and reached the 78th percentile in our industry sector.
EcoVadis is a global business Sustainability Ratings company, well known in the medical industry. EcoVadis operates an evidence-based online platform, providing sustainability ratings and allowing companies to assess the ESG performance of their global suppliers. The EcoVadis Rating covers a broad range of non-financial management systems including environmental, labor & human rights as well as ethical and sustainable procurement. Each company is rated on the material issues as they pertain to their company’s size, location, and industry.
Elcam is committed to maintaining good corporate citizenship by actions related to different aspects of ESG:
Social responsibility in Elcam means promoting diversity and inclusion in the workforce and providing equal non-discriminatory working opportunities. Our employees come from 33 countries across the world and from 5 main religions and ethnicities (Jews, Christians, Muslims, Circassians and Druze). In our Israeli facilities alone, we employ workers from 72 different communities from all around the northern part of Israel. We currently have 47.5% women employees and 37.5% women in managerial roles.
Social responsibility also means being involved and taking an active part in promoting social issues in our communities and especially creating role models for future generations. We do so in a variety of ways, such as donations to local hospitals and establishments in need in Modena, Italy during the COVID19 pandemic, or investing in one of the high schools near our Israeli facility, where together, we’re building an innovation lab for research and promoting technological education through different activities. Our Italian facility is one of the founding members of the ITS FOUNDATION — New Technologies of Life, which carries out free two-year and post-diploma courses to train senior technicians in the fields of design, production and quality assurance of the biomedical sector, bringing highly specialized skills and innovation capacity to companies.
Environmental responsibility in Elcam comes in many ways — recycling plastics and cartons, maximizing efforts in order to save energy by adopting innovative technologies, and employing natural gas and solar energy as environmentally-friendly energy generation for self-consumption in our facilities. We also encourage our employees to be more environmentally friendly through recycling, reducing use of plastics, sustainable mobility and more.
Governing responsibility means being obligated to high standards of Ethical and Business Conduct. Elcam operates both internally and externally in a fair and non-exploitive manner, according to the laws and regulations in all the countries we operate in. Our managers and employees abide by a comprehensive set of internal procedures and guidelines which are consistent with our commitment to acting with integrity in all that we do. As part of Elcam’s ongoing improvement processes, we are currently refining our code of conduct. Furthermore, in order to promote the ESG concept among our suppliers and help them do better for their stake holders, we also ask our major suppliers to acknowledge our code of conduct for suppliers.
Elcam believes that through our ESG responsibilities, we can enhance collaboration with colleagues, strengthen relationships with customers, partners and society, and create added value.
Elcam’s Ecovadis Silver Medal
Product Customization
Amir Bohadana I 14.07.2021
Customizing products to fit different needs of various applications and usage is a basic requirement of most manufacturing companies and in particular medical device manufacturers.
Medical device companies are consistently searching to develop new solutions for improved procedures and more effective patient treatment. The innovation of new solutions often requires customized devices and components which in many cases are small however essential part of the whole new product development.
Some manufacturers use in-house customization capabilities while others outsource projects to companies that have expertise in specific product lines, especially when it comes to smaller components and devices. To make it feasible for a company to invest such efforts in a specific component or a production line, it must be a company that focuses on a relative niche product line.
When it comes to medical product customization being able to customize products is good, but to truly be a significant partner in developing new systems, one needs to be familiar with the clinical area, have vast experience and internal knowledge in product customizations.
Furthermore, product customization processes require significant documentation in order to receive regulatory approval. In the medical device field this requirement is trifold – as each change requires regulatory approval and documentations according to ISO standards, biocompatibility, shipping tests and more.
Elcam Medical is a world class producer of disposable medical devices and accessories for the OEM market, and a provider of innovative solutions for specialized flow control needs.
Being an OEM company, the capability to customize our products to fit new developments is one of our most important assets.
Elcam’s focus on disposable components for OEM, enables us to invest a lot of efforts in this line which led us to having the broadest portfolio of stopcocks, manifolds and connectors for IV and drug delivery, with more than 1000 SKUs for the stopcocks line alone.
Elcam does not work as a subcontractor. We treat our customers as partners and share our extensive knowledge in product development and our long-standing clinical know-how gained in over 40 years of experience. The Elcam teams know how to recognize market needs and recommend solutions, making us a significant partner in the development process.
Furthermore, as a true OEM company, Elcam’s quality assurance and regulatory affairs department provides customers with comprehensive regulatory support including all the required documentation.
View several examples of Elcam’s customized products
Partnership is an integral part of Elcam’s business philosophy and actions as we believe in building and nurturing long term, stable relationships with our customers and partners, as well as in sharing risks, knowledge, and expertise, so we can grow and prosper together.
Partnering & Business Development
Partnering & Business Development
Growth is one of Elcam’s values and it means for us being bold and innovative in new products, services and solutions. Elcam is invested in product innovation with more than 20 new ideas reviewed annually and over 7000 manhours devoted to screening new projects. We innovate together with our customers and partners in an effort to provide real solutions to real problems.
Understanding the needs and requirements for safe and effective medical care requires the expertise of healthcare professionals, clinicians as well as clinical specialists of medical device companies. Elcam uses its close cooperation with OEM customers, key clinicians and inventors to explore the medical market on an ongoing basis in order to stay up-to-date and committed to the medical market evolving needs and trends. Explore our review and development processes

.f_p{ transform: translateX(-80px);}
@media only screen and (max-width: 750px){
.f_p{ transform: translateX(0);}
}
Elcam strives to enhance patients’ and medical teams’ safety. The two following examples show how our innovation is rooted in the unmet needs of the medical fields we operate in
SafeT™ Product line
SafeT™ Product line
The potential for Environmental Stress Cracking (ESC) is a well-known concern when plastics are used in medical device components. The use of lipids and other aggressive solutions can initiate microcracks in the polymer and compromise the material’s mechanical integrity. Adverse events such as medication discontinuation, blood loss, air and bacteria ingress, as well as exposure of clinicians to hazardous drugs and bodily fluids, are all potentially dangerous events that may result from ESC and need to be prevented as much as possible by safety guidelines and smart products. One way to decrease the incidence of ESC is to use more resilient raw materials as did Elcam with its SafeT™ product line.
Elcam Medical’s Tritan™ integrated SafeT™ Stopcocks and Connectors provide safer treatment through enhanced lipid and chemical resistance.
Tritan™ is a new-generation copolyester that has been shown in bench-top testing to be resistant to a large spectrum of medical fluids such as oncology drugs, lipids, drug carrier solvents, and nutrition.
The SafeT™ stopcocks have been tested and proven to be resistant to 10 different drugs including lipid and other aggressive substances and IPA (isopropyl alcohol).

NRFit Products
NRFit Products
Luer connector misconnections are under-recognized, but common and potentially dangerous events. The objective of the ISO 80369 Standards is to prevent these adverse events and improve patients’ safety. The ISO 80369-6:2016 Standard, is part of the 80369 series and affects devices for neuraxial applications such as epidural devices, pain medication pumps and their administration sets, regional anesthesia catheters and needles. Elcam has taken a proactive approach to the implementation of the new ISO 80369 standard regulations and currently has products that comply with the different parts of the standard (-3, -6, -7). Our Neuraxial (NRFit) products are compliant with the new ISO 80369-6 standard. They include the NRFit stopcocks and both male and female ISO 80369-6 connectors. The connectors are also available with clear Tritan™ material from Eastman, providing further advantages of improved lipid resistance and the benefits of not containing BPA.
Read more
Team news
Team news
Tomer Gil, of Elcam Business Development and Marketing team is relocating to our US office in Hackensack, New Jersey. Tomer has been with Elcam since 1993, first as an engineer in R&D department and during the last 15 years as a Marketing Director. Tomer will continue to fulfil all his former tasks from the US and will also take charge of US business development activities. Join us in wishing him a smooth transition and much success in his new role

Notification regarding discontinuation of raw material
Notification regarding discontinuation of raw material
Last year LyondellBasell announced the discontinuation within 2023 of its polypropylene material Purell HP 371-P.
Over 400 codes from Elcam Italy’s product portfolio are involved. Elcam Medical started and almost completed a replacement program, with the Borealis polypropylene Bormed RF830MO. For some product families, like Caps and Clamps, Elcam will offer further alternative with the new LyondellBasell polypropylene Purell HP 373-P.
The replacement of the materials will take place throughout 2023.
Elcam strongly recommends our customers who use our products made of Purell HP 371-P to anticipate this changeover at earliest possible. This may enable us to offer the same components made of alternative equivalent polypropylene materials.

Stay safe and healthy
Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
PDA Universe of Pre-filled Syringes
18-19 October
Palm Springs, California
Booth 308
PODD Partnership Opportunities in Drug Delivery
24-25 October
The Westin Copley Place, Boston
CPHI 2022
1-3 November
Frankfurt, Germany
Booth 30J43
Compamed
14-17 November
Duesseldorf, Germany
Hall 8a Booth E28
Medtec China
7-9 December
Shanghai, China
Hall B1 Booth H003
Elcam believes that being a socially responsible medical device company in today's world requires a holistic attitude, a balance between providing the best clinical outcomes for patients and caregivers, while maintaining a positive impact in all areas comprising the company, including workforce, environment, economics and community.
Customization and Tailor-Made Adjustments
MedAccred
Elcam has recently received the MedAccred accreditation for plastic injection molding processes for its Israeli sites, and has become the first company in Israel to be awarded this accreditation.
MedAccred is an industry managed, consensus-driven approach for ensuring critical manufacturing process quality throughout the medical device supply chain. In today’s world of global manufacturing, the supply chain is multi-tiered and geographically remote, making oversight challenging and costly. To prevent output deficiencies, critical processes and products must be validated during manufacturing to prove that they are fit for purpose, satisfy customers’ and regulatory requirements and reduce overall risk. The MedAccred accreditation is unique because it is industry driven and managed. Its subscribers are the top tier medical device companies such as Bausch Health, Baxter, Becton Dickinson, Boston Scientific, Edwards Lifesciences, Johnson & Johnson, Medtronic, Philips, Roche Diagnostics and Stryker. These companies fund and manage MedAccred, determine audit criteria, have full access to audit findings, and determine who is granted accreditation.
Israel Dembus, Elcam’s Chief Quality & Regulatory officer shared: “Elcam decided to obtain the MedAcrred accreditation based on our understanding that this accreditation is a growing trend in improving the medical device industry quality standards, which will help us better serve our customers and partners. The accreditation validates that we maintain the highest quality standards for our products and ultimately helps assure patient safety.”
Elcam intends to continue the accreditation process and to receive accreditation for all applicable production processes in all worldwide sites during the next 2 years.
MedAccred certificate
Ecovadis
Ecovadis
Elcam Medical was recently awarded a Silver Business Sustainability Rating by Ecovadis.
EcoVadis provides holistic sustainability ratings service of companies. The EcoVadis Rating covers a broad range of non-financial management systems including environmental, labor & human rights, ethics and sustainable procurement impacts. Each company is rated on the material issues as they pertain to their company’s size, location and industry. These evidence-based assessments are refined into easy-to-read scorecards, providing zero to one hundred (0-100) scores, and medals (bronze, silver, gold & platinum), when applicable. Additionally, the scorecards provide guidance on strengths and improvement areas, which the rated companies may use to focus their sustainability efforts and develop corrective action plans to improve their sustainability performance.
Hadas Eisenberg, Elcam’s Sustainability officer explained: “Elcam chose to be rated by Ecovadis as part of our belief in the importance of ESG (environmental, social and governance) and sustainability as well as our approach to maintain and keep improving high level customer experience.
We are proud to share the recent silver rating we received from Ecovadis. Elcam scored in the 78th percentile of all companies in our industry that were rated by Ecovadis and we aim to continue and improve our score over the next years”.

Closed stopcocks contribution to reducing infection rates
Closed stopcocks contribution to reducing infection rates
In a recent article (February 2022) of the APSF (Anesthesia Patient Safety Foundation) Newsletter, Dr. Elliot S. Greene wrote about ‘Challenges and Solutions for Reducing Infection Risks When Accessing Vascular Catheters’ and discussed the contamination and infection risks when using disinfected DNCCs (Disinfectable Needleless Closed Connector) and OLS (Open Lumen Stopcocks). In his notes he wrote: “The current literature supports that disinfected DNCCs should be used instead of OLSs based on the following premises: the documented overall lower contamination and infection risks of disinfected DNCCs compared to OLSs and the recent SHEA recommendations that “stopcocks used for injecting drugs should ideally be closed with needleless injection ports.” To reduce infection-related patient risk, vascular catheters used for medication or fluid administration, or blood withdrawal, should be routinely accessed via either disinfected DNCCs or via disinfected DNCC-stopcocks. Compliance with disinfection is essential. For DNCC-stopcocks, the DNCC should preferably be bonded to the stopcock injection lumen to eliminate removal and bypassing the DNCC. Current studies support that blood samples from arterial tubing sets be obtained via disinfected DNCC-stopcocks instead of OLSs. Manufacturers should supply IV and arterial transducer tubing sets with DNCC-stopcocks instead of OLSs and there should be DNCC-stopcocks made available as single packaged items.”
Elcam’s Closed swabbable Stopcock and Marvelous™ Stopcocks are both DNCC stopcocks that present many advantages to both clinicians and patients for helping reduce infection risks.
The Closed Swabbble Stopcocks are DNCCs as described by Dr. Green – Stopcocks integrated with a swabbable luer activated valve (i.e, disinfectable needleless closed connector) allowing for maintenance of a fully closed system through the entire use of the stopcock, as well as eliminating needle stick injuries when using a stopcock. This integration also reduces the stopcock’s residual volume in comparison to standard stopcock with attached LAV cap.
As for the Marvelous™ stopcock– this unique product carries more advantages as in addition to being closed & swabbable it also has a proprietary flow channel feature specifically designed for IV administration and pressure monitoring applications, allowing to continuously flush drugs and blood residuals, without the need to expose the system to the open air, assuring minimal residual volume and further reducing infection risks.

Back to face to face
Back to face to face
It looks like the Covid – 19 is moving away and Elcam is very happy to be able to return to in- person meetings.
Over the past 2 years many of us have spent a lot of time apart, away from our normal lives. For Elcam, our business model has always been relationship-driven, but the pandemic largely put a halt to in-person customer meetings. Just like our customers, how we are able to meet has changed dramatically since the beginning of Covid-19.
In some cases, the shift wasn’t a totally bad thing. We’ve all learned how to use Zoom, Teams, Webex and other communication tools, and once everyone got comfortable with the technology, it was beneficial being able to meet with customers virtually. Now as we are beginning to see the end of the pandemic, we are all trying to understand what meetings are supposed to look like in a post-pandemic world. What will the new normal look like for businesses like ours that used to count on face-to-face interaction as a core part of how we work?
It’s likely we will not completely return to pre-pandemic modes of conducting business – the conveniences of modern technology are just too great to abandon entirely. On the other hand, we do not expect our business to stay strictly virtual. We expect a hybrid approach to communicating with our customers, taking what we’ve learned during the last 2 years and applying it to our future business practices.
We at Elcam are always looking for a return to personalized service and we are looking forward to getting back to seeing our customers face to face. As your situation changes, please contact your Elcam representative to schedule a meeting and we hope to see you face-to-face in the near future.

Stay safe and healthy
Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
Medtec China
31 August – 2 September
Shanghai, China
Hall 1 Booth 2H003
PDA Universe of Pre-filled Syringes
18-19 October
Palm Springs, California
Booth 308
PODD Partnership Opportunities in Drug Delivery
24-25 October
The Westin Copley Place, Boston
Compamed 2022
14-17 November
Duesseldorf, Germany
View several examples of Elcam’s customized products
Elcam is investing 1.5 millionִ$ in Serrenno Medical for development and manufacturing of a unique urine output monitoring device, the Sentinel™.
Read moreElcam continuously strives to improve customers’ experience in many aspects of our work. In this edition we are happy to highlight the company's customization capabilities, and the way they are expressed in our latest product offerings - Stopcock with Tube assembly and Disposable Pressure Transducer (DPT). We are also delighted to introduce several new team members that are part of the renewed sales department structure and the results of our annual customer satisfaction survey.
Customization and Tailor-Made Adjustments
Customization and Tailor-Made Adjustments
Elcam products can be customized according to customers specific needs, offering the company’s expertise, world class equipment, and production capacity for custom made products for you. Our customization capabilities are broad and include unmatched configurations, dimensions, flow rates, colors, variety of high-performance materials and more.
Elcam Medical is committed to understanding your unique requirements, applying our 40 years of experience and advanced manufacturing capabilities to every project, in order to design, develop and manufacture comprehensive solutions for your customers need.
Michael Segev, Elcam’s Chief Technology officer, shared: “When it comes to customization, we combine your idea with our in-depth product knowledge and expertise. Our Design and Development Engineering team through its vast experience, creativity and application of up-to-date engineering tools, is qualified to give the best advice and support in cost-effective designs, raw materials, product features and specifications, while the Process Engineering team transforms prototypes into manufacturable, cost-effective, validated devices, as well as developing and adjusting specially designed production lines”.
Over the last 5 years Elcam has developed 150 customized products. The company’s renowned stopcocks that are now available with tube assembly in a variety of dimensions as well as the low-profile disposable pressure transducers (DPT), constitute just a few examples of Elcam’s capabilities to provide tailor made adjustments.

Stopcock with Tube Assembly
Stopcock with Tube Assembly
As part of Elcam’s continuous efforts to expand product range to best answer customers’ needs, Elcam is now offering its standard and Marvelous™ stopcocks with a variety of extension tube lines as cost effective sub-assemblies.
Elcam offers the widest variety of tube fitments for bonding, off the shelf or tailor made, that together with Elcam’s highly automated processes ensure consistent production of high-quality products.
Available customization options include different tube lengths and Stopcock configurations, male luer lock connectors and optional connector covers including Elcam’s unique Safe2 Rotator™ connector as another optional customization
Read more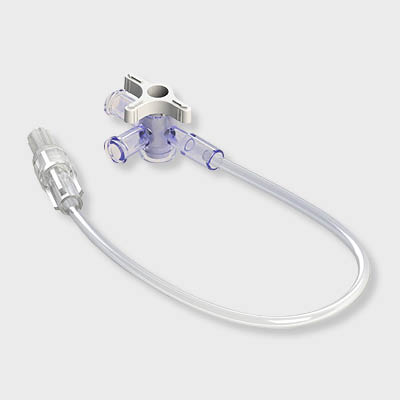
Flat (Low- Profile) DPT
Flat (Low- Profile) DPT
The new low-profile DPT that is gradually replacing Elcam’s prior DPT was designed per request from the market to be more suitable for applications that do not require the use of a flush-device. The low profile is more elegant, intuitive and easy to use. The new low-profile transducer offers many customization options including different luer configurations as female or male luers, tube sockets or bonded stopcocks, as well as variety of connector types and also optional logo engraving or printing. The DPT continues to present all the benefits of the older version:
Ease of use
Full visibility of the flow path
Easy and safe priming and debubling
Improved luer locks – utilizing Elcam’s “ramp lock” patent for excellent connectivity
Reliability & Safety
Elcam’s High quality standards
The DPT is suitable for any pressure monitoring applications for which Flush Device is not required
Read more
New Sales department structure and team members
New Sales department structure and team members
Elcam sales department has recently made some organizational changes in an effort to better serve our customers’ needs. The Sales department under the Management of Amir Bohadana has been divided into 3 groups: Product management – Stopcocks & Manifolds (Catalina Gutman), Patient Monitoring and Interventional (Gal Halperin) and Elcam IV &Dialysis Components (Gianluca Mariotti); Territory management (US- David Landspurg, Europe – Gianluca Dispenza, Asia, LATAM, ME & Africa – Alon Gamliel) with corresponding specific local account managers; and of course, our dedicated Customer Service teams in all territories.
We would like to introduce our new team members:
Gal Halperin is Elcam’s new Patient Monitoring & Interventional Procedures Product Manager. Gal has a BA in economics and statistics and MA in Business administration. Gal has joined Elcam 4 years ago as a cost accountant and is familiar with Elcam’s products and customers. He is a member of Baram and has deep roots in Elcam as his parents and even grandparents are veterans of Elcam. Gal shared: “I’m very excited about my new position. I love a good challenge and I look forward to deepen my knowledge and learn the clinical background of the products. I believe that this knowledge and good relations are key factors for Elcam’s and our customers’ success”.
Alon Gamliel is the new territory manager for Asia, Middle East & Latin America. Alon has 20 years’ experience in the international market and 15 years in the medical market. His latest positions were at Medispec and at Schultz Medical in England as a commercial manager for about 5 years. He has a B.Sc. in Electrical engineering. Alon says: “I believe that Hard work can divert falcons in flight. Working at Elcam is a dream come true, of challenges throughout the company, working with professionals, who feel that everyone is an integral part of the company as a whole.”
In November 2021, EMI welcomed Abe Santrich to the team in the role of Logistics Customer Service Coordinator. Abe’s background is in Logistic and Import Management and his skills and abilities will be a major contributing factor in continuing the exceptional customer service and support our customers expect. Abe stated: “I am grateful to be given the opportunity to be part of this team and be able to enhance my experience by working together with each team member.”
Saar Maoz is our new Customer Service for the USA and Latin America territories. He joined Elcam after working at Apple Inc (London) as a customer service team leader and in the last 3 years as the Deputy CEO of Israel AIDS Task force, where he was responsible for both human resources and advocacy on a governmental level. Saar moved to Baram in 2020. Saar tells us: “I believe in people and that getting communication flowing is the way to create success in abundance. I would love to see Elcam continue to grow and be a safe sustainable environment that promotes diversity and inclusion”.
Einav Gutman joined the Customer Service team in Israel a year and half ago after previous roles in agriculture, studying economics and management, and working as an accountant in Baram’s Agricultural industry. Einav is a proud father of 3 teenagers. He loves sports and manages Braam’s Sports department. Einav shared: “I had the great opportunity to join Elcam’s team. I look forward to continue working with Elcam customers, to provide the best solutions to their needs”.

Customer Satisfaction Survey
Customer Satisfaction Survey
Like every year we have sent you the Elcam’s annual customer satisfaction survey in December and have recently finished summarizing its results.
We would like to thank all of you that took the time to answer the questions and help us with Elcam’s consistent efforts to improve its services.
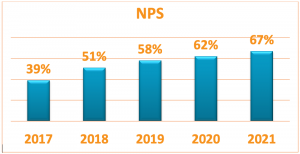
Despite the obvious difficulties dealing with the ongoing pandemic and its continued impact on the global logistics crisis as well as raw material shortages and price increases, Elcam is excited to share the feedback received from the 2021 customer satisfaction survey that shows a high level of satisfaction from Elcam services and products quality in general, and from the reliability of supplies specifically.
The Net Promoter Score (NPS score) measured annually representing the customers loyalty levels (a positive score is considered good, and an NPS of +50 excellent), further improved in 2021 and received the best score we had seen so far. Amir Bohadana, Executive Sales Director, elaborated: 2021 was one of the most challenging years for keeping our services at high level in light of the world logistics crisis. We were happy to see from the survey results that our efforts have been successful. We are aware that this crisis is not behind us yet and that we will need to try even harder to keep ranking above 50 in the following years as well”.

Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
MD&M West
12-14 April
Anaheim, California, USA
Booth 2339
Medtec China
31 August – 2 September
Shanghai, China
Hall 1 Booth 2H003
View several examples of Elcam’s customized products
Elcam is continuously working to enhance both our product offerings and support services. In this edition of our newsletter, we’re highlighting Elcam’s Interventional Cardiology & Radiology portfolio and capabilities. You’ll also find updates on our new facility in the Dominican Republic and an invitation to follow us on Elcam’s social media channels.
Interventional Cardiology & Radiology
Interventional cardiology and radiology (IC&R) procedures require specialized devices and accessories distinct from those used in other medical settings. Furtheremore, these tools are often in continuous use and held directly by physicians throughout the procedure, making ergonomic design a critical factor for comfort and efficiency.
Elcam Medical offers a comprehensive range of IC&R-focused accessories developed in collaboration with interventional experts. Our portfolio includes high-pressure stopcocks, manifolds& rotators, hemostatic valves, and disposable pressure transducers – all engineered to meet the rigorous performance and safety standards of interventional applications.
Following are some great examples of Elcam’s Interventional line offerings
Explore our Interventional solutions
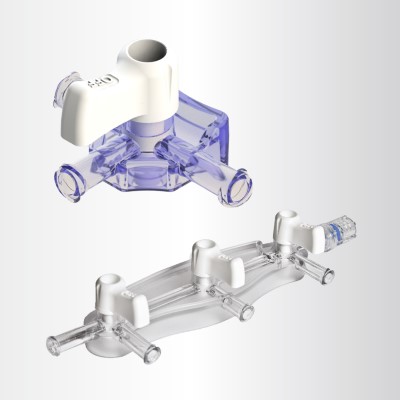
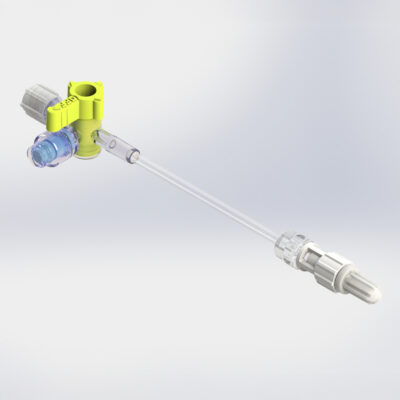
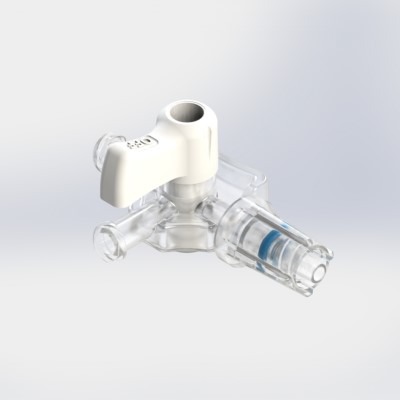
High Pressure (HP) Stopcocks & Manifolds
The HP+ Stopcock, compatible with Gamma and E-Beam sterilization, is the latest addition to Elcam’s High Pressure (HP) Stopcock and Manifold line. It offers all the advantages of our legacy HP products, now combined with advanced sterilization compatibility. This ensures the highest level of safety for high-pressure applications, without compromising ease of use.
Key Advantages of Elcam’s HP Stopcocks and Manifolds:
- Ergonomic handle designs for enhanced workflow
- Wide product variety to suit diverse clinical needs
- Multiple customization options
- HP and HP+ Stopcocks: Designed for CathLab use, with pressure durability of up to 1,200 psi
- HP Manifolds: Withstand up to 800 psi—50% higher than most comparable solutions, ideal for cardiac angiography and angioplasty procedures
Discover the new HP+
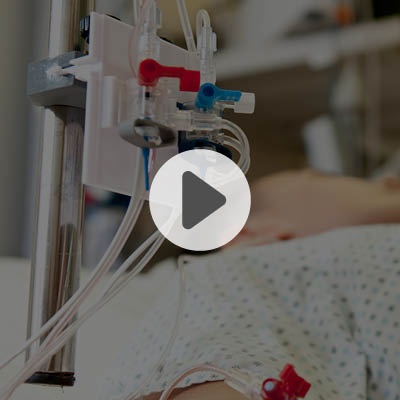
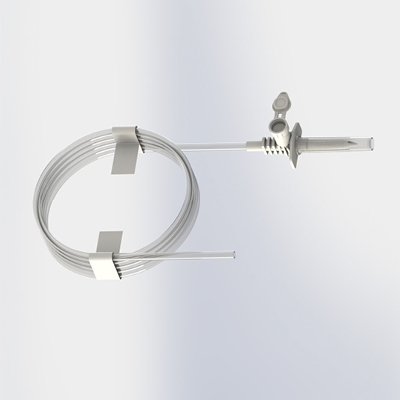
Y-Click™
Elcam’s ergonomically designed, user-friendly hemostatic valve engineered with insights from leading interventionists. The Y-Click features a unique three-stage lever system and offers key benefits for cardiologists:
- One-handed operation that frees the other hand, enhancing procedural efficiency
- Audible clicks that confirm valve positions without requiring visual checks
- Advanced blood loss control, including a unique Semi-Open mode that reduces bleeding while maintaining device maneuverability
Disposable Pressure Transducer
Elcam’s low-profile Disposable Pressure Transducer (DPT) is specifically designed for applications that do not require flush devices, making it an ideal choice for interventional cardiology and radiology, where a streamlined design and reliable performance are essential.
The DPT offers full flow path visibility, easy and safe priming and debubbling, Elcam’s patented Ramp Lock™ technology for secure connectivity, and multiple customization options to meet diverse clinical needs.
For more information about our DPT

New facility update
Elcam’s new manufacturing facility in the Dominican Republic is progressing on schedule. The building envelope is expected to be completed by year-end, with most exterior walls finalized by May.
Next, we’ll begin constructing cleanrooms, assembly and injection areas, a logistics center, and offices, aiming for full operations by September 2026.
This expansion supports Elcam’s commitment to better serve our North American clients and ensure more reliable supply chains, while also creating 160 new jobs in the local community.
Elcam on Social – Follow Us!
Stay connected with Elcam Medical through our social media channels.
Every week, we share insights into our products, manufacturing capabilities, and the latest company updates from around the globe. Whether you’re a healthcare professional, partner, or industry expert, our platforms offer a closer look at how we’re advancing medical flow control solutions.
Follow us on LinkedIn, Facebook, Instagram, and X to stay informed and inspired. Join the Elcam community today!

Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
Medtec China
24 – 26 September
Shanghai, China
Booth #2D001
Elcam continuously strives to improve product offerings and supporting services. This edition of our newsletter highlights Elcam’s tube assembly capabilities including the Marvelous™ with tube and spikes with tube. We share the results of our annual customer satisfaction survey, update on the upcoming EU MDR regulation, and introduce our new Chief Technology Officer.
Tube assembly Capabilities
As part of Elcam’s continuous efforts to expand product range to best answer stopcocks needs, Elcam is offering its stopcocks with a variety of extension tube lines as cost effective sub-assemblies.
Elcam offers the widest variety of tube fitments for bonding, or oversizing, off the shelf or tailor made and also offers its professional support in building and tweaking your bonding process.
Following are some great examples of Elcam’s tube assembly capabilities and offerings
Learn more about reducing plasticizer-induced stress cracking
Marvelous™ with tube
Optimize inventory management and enhance safety with the Marvelous™ stopcock and extension tube. The innovative design of Marvelous minimizes contamination risks and streamlines medication delivery. The inner channel and responsive valve provide superior protection against contamination, air entry, and residual medication. The extension tube offers greater flexibility and easy access without frequent disconnections further minimizing infection risks.
Learn more about the Marvelous
Stopcock with tube
Elcam’s Stopcock with extension Tube line offers ultimate convenience and efficiency while maintaining the top-quality standards customers have come to expect. Elcam’s wide range of stopcocks and tubes allows you to customize your setup to fit your specific needs.
Explore our stopcocks with tubes varietySpike With Tube
Elcam is expanding its tube assembly capabilities to include Spikes with tubes which can seamlessly integrate with existing IV setups. Elcam’s spikes are designed to meet a variety of applications including IV, drug transfer and chemotherapy. The extension tube provides greater flexibility, allowing easy access as well as reducing inventory management costs while maintaining the highest quality standards.
Learm more about our spikes
Customer satisfaction survey
Elcam recently completed the analysis of its annual customer satisfaction survey, which was sent out in December. We extend our gratitude to all who took the time to provide valuable feedback, which supports Elcam’s continuous efforts to enhance our services.
Amir Bohadana, Elcam’s Chief Sales Officer concluded: “We were happy to see that the efforts we have invested during 2024 to uphold our promise of consistent delivery and support have resulted in continued customers satisfaction in responsiveness time, on-time delivery, quality and technical support. We are aware there are still areas where we need to improve as we observed a decrease in NPS score, which reflects the customers loyalty levels and aim to improve the score in the upcoming years”.

EU Regulation 2023-607 MDR News
As you may be aware, the European Regulatory Agency ECHA has set a deadline for the use of DOTE in products distributed in the European Market. This is a forced change that applies to all operators involved in the supply chain of plastic components, from suppliers of chemicals (substances) to manufacturers of products containing such chemicals.
The deadline for discontinuing the use of DOTE is May 1, 2025. After this date, Elcam will no longer be allowed to purchase raw materials, produce, or supply products containing DOTE. The new rule prohibits the use of DOTE even at a concentration below 0.1% w/w. All raw material suppliers have informed us of their decision to comply with the deadline and phase out all products containing the DOTE stabilizer.
To mitigate the impact of this discontinuation, Elcam has already replaced affected part numbers with new, fully compliant alternatives. These alternatives meet the same or improved quality and performance standards and are available for sampling upon demand. Our Customer Service team has already provided a comparison table of old and new part numbers to ensure a smooth transition. Elcam will be happy to support you in this transition and with any documentary requests.
Contact our customer service for more details
Team News
Elcam is delighted to announce the appointment of Matan Gedulter as our new Chief Technology Officer. Matan holds a Bachelor of Science in Biomedical Engineering and a Master of Business Administration, bringing 20 years of experience in medical device R&D. Over the course of his career, he has driven innovation in developing devices across multiple clinical fields and is the inventor of numerous patents in the industry.
Matan shared his excitement about joining Elcam, stating: ‘I am enthusiastic to join Elcam’s professional, experienced leadership team and to have the opportunity to make an impact on the medical device industry on a global scale. At Elcam, I will focus on advancing our portfolio of high-quality, state-of-the-art medical devices and components, reinforcing Elcam’s commitment to delivering trusted solutions that enhance patient care worldwide, and tailoring customized, optimized solutions to increase our customers’ value proposition.”
Join us in welcoming Matan to the Elcam family
Contact Matan

Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
Medtec Japan
9 – 11 April
Tokyo, Japan
Booth 1503
Elcam continuously strives to improve product offerings and supporting services. In this edition of our newsletter, we highlight Elcam’s solutions for drug delivery and the stopcock with tube assembly offering, as well as share updates of Elcam's current situation, and valuable information for preventing invoice fraud
Elcam Stands Strong– Update #10
We are happy to share some hopeful and encouraging news regarding the situation in our region.
A ceasefire agreement has now been reached, and we are optimistic that this will bring an end to the conflict in the northern region of Israel.
Moreover, we believe this positive development will pave the way for a resolution and an end to the war in Gaza in the near future.
While Elcam has successfully maintained routine operations throughout the past year, this significant change reduces the need for many of the precautions we had implemented to ensure business continuity.
We are heartened to see that citizens in the northern border areas are beginning to return to their homes, a sign of renewed stability and normalcy in the region.
We are grateful for the resilience of our team and the unwavering support from our partners, which have been instrumental in navigating these challenging times.
We remain committed to delivering the quality, reliability, and service you expect from us, and we are hopeful that these recent developments mark the beginning of a more peaceful and stable future for all.
Thank you once again for your continued trust and partnership. If you have any questions or require further assistance, please don’t hesitate to reach out to us.

Elcam Drug Delivery Devices (E3D)
The demand for devices that enable parenteral drug injections outside healthcare settings is increasing, driven by the rise of biologic drugs and their biosimilars with compound annual growth rate of 17.6%, for long-term treatments of chronic conditions requiring self-administered subcutaneous injections.
With most patients needing weekly injections, the potential for environmental waste highlights the need for advanced delivery systems. Pharmaceutical companies must focus on auto-injectors that ensure reliability, minimal waste, cost-effectiveness, and ease of use, while also reducing environmental impact and production costs.
E3D is committed to meeting these challenges, offering drug delivery devices designed for reliability, sustainability, and efficiency, enhancing both patient care and environmental responsibility.
Following are 2 devices that represent this commitment
Explore more in the following link

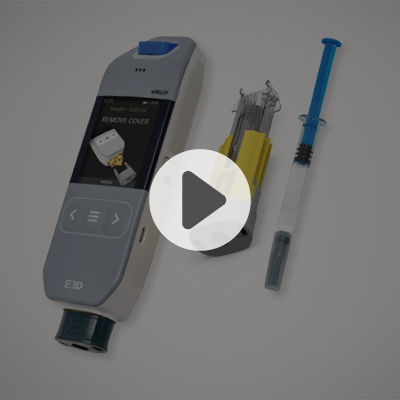
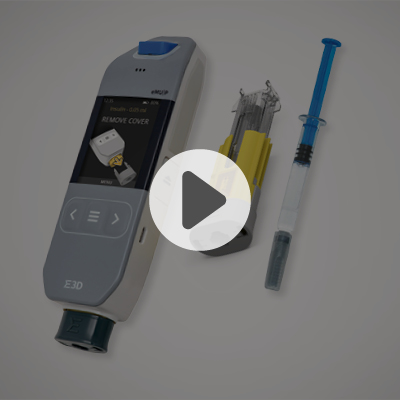
Flexi-Q mMU
The Flexi-Q mMU is a mechanical, multi-use auto-injector system that combines a reusable device with a single-use cassette containing a drug-filled syringe.
The mMU simplifies the injection process significantly compared to other reusable auto-injectors. Its intuitive “Click ‘n Go” mechanism reduces the traditional 11-step procedure to just 4 handling steps, lowering the risk of errors and making self-injection easier and less stressful.
Adopting E3D’s reusable auto-injector for twice-weekly use over a year allows for replacing 104 standard auto-injectors with one reusable device and 104 disposable cassettes, leading to a 60% reduction in environmental impact and 80% cost savings.
Addressing both patient needs and sustainability goals, the Flexi-Q mMU represents a forward-thinking solution for the future of healthcare
Explore moreFlexi-Q eMU-P
E3D’s Flexi-Q eMU-P is an advanced electro-mechanical multi-use auto-injector system designed for administering drugs in pre-filled syringes. It offers an innovative and sustainable solution for the self-administration of biological drugs, aiming to improve patient care by making it easier, safer, and more efficient.
The Flexi-Q eMU-P integrates a reusable device with a disposable cassette providing the waste and cost savings benefits of multi-use design. Its advanced features bring a host of benefits enhancing treatment safety and efficiency: step-by-step on-screen guidance, full needle protection, and precise dosing, coupled with user-friendly design including advanced connectivity options, enabling seamless data integration.
Flexi-Q eMU-P stands out as a sustainable, state-of-the-art device in modern healthcare.
Read more
Stopcocks with Tube assembly
Elcam offers cost-effective stopcocks with extension tube lines, now additionally available with the innovative Marvelous™ closed needleless minimal residual volume stopcock. These mini-set options can also be fitted with male Luer lock connectors and covers, including the unique Elcam Safe2 Rotator™ connector for additional customization.
These versatile sub-assemblies are available in a variety of configurations to suit the needs of various applications, such as IV lines, introducers, central venous catheters, and pressure monitoring lines.
Elcam’s Stopcock with Extension Tube Line combines convenience and efficiency while upholding the high-quality standards customers expect. With our broad selection of stopcocks and tubes, you can easily tailor your setup to meet your specific requirements.
Learn more about tube bonding
Invoice fraud
Invoice fraud is unfortunately quite prevalent and can be difficult to detect. As a result, it often occurs over an extended period, leading to substantial financial losses for businesses—sometimes amounting to thousands, or even millions, of dollars. According to data from the Association of Certified Fraud Examiners (ACFE) in their Occupational Fraud 2022: A Report to the Nations, invoice fraud and similar billing schemes were among the most frequently reported types of asset misappropriation.
At Elcam, we are taking all necessary precautions to prevent such fraud, and we urge our customers to stay vigilant. One of the most effective ways to prevent invoice fraud is to ensure that whenever there is a notification about a change in the recipient’s bank account, it is verified through a video call or a direct phone call. To help raise awareness, we encourage you to explore more about this issue through the link above and the following blog: Invoice Fraud: How to Identify Fake Invoices & More
Read more
The Elcam team wishes you and your loved ones a joyful holiday season and a peaceful, healthy and prosperous year in 2025
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
Pharmapack
22 – 23 January
Paris, France
Hall 7.2 | Booth H17
MD&M West
4-6 February
Anaheim, California
Booth 2449
Elcam continuously strives to improve product offerings and supporting services. In this edition of our newsletter, we highlight Elcam’s solutions for Interventional Cardiology & Radiology, our Safe2 Rotator, as well as share updates of Elcam's current situation, Ecovadis 2024 rating and introduce new team members.
Elcam Stands Strong– Update #9
As we continue to navigate the ongoing conflict in our region, we continue to provide you with an update on our operations and the proactive steps we have taken to ensure uninterrupted service.
Despite the tensions in the northern part of Israel, Elcam Medical’s facilities remain fully operational. We are pleased to report that there has been no disruption in our supply chain, and we assess the probability of future disruptions as low. We are committed to maintaining this stability and are prepared for various contingencies.
To remind you of the measures we have implemented to mitigate any risk of supply disruption:
Inventory Redistribution: We have strategically moved inventory to our logistic centers in Dalton and our facility in Carpi (MO), Italy. This is in addition to our existing logistics center in North America, ensuring a diversified storage solution that supports continued supply.
Flexible Production Capabilities: We have enabled the production of similar products at both our Dalton and Bar’am facilities, enhancing our flexibility and responsiveness to demand fluctuations.
Government Support: Elcam is designated as a “vital facility” by the Israeli government. This status affords us full support to keep our operations running smoothly, including priority in logistics and additional security measures.
We remain hopeful for peaceful times and are vigilant in our efforts to ensure that our operations are not affected. Your trust in our ability to deliver high-quality medical components during these times is greatly appreciated, and we do not take it lightly.
Thank you for your continued partnership and understanding. We will keep you informed of any significant changes and are here to answer any questions you may have.

Interventional Cardiology & Radiology
To enable interventional cardiology and radiology procedures to be carried out smoothly and efficiently, Elcam has developed easy-to-use, ergonomic, and cost-effective solutions for use in interventional applications.
Designed with experts, our solutions include Y-Click connectors for PTCA applications, disposable transducers, high-pressure stopcocks, manifolds and rotators, all specially suited to the needs of cath labs and inetrvenional suits.
Following are a a few excellent examples
Explore our Interventional product line
New High Pressure Gamma and E-Beam Compatible Stopcocks
By using advanced materials and introducing unique design features, Elcam has created the new Gamma and E-Beam Compatible HP stopcock (HP+ stopcock).
The HP+ stopcock enjoys all of the advantages of Elcam’s legacy HP stopcock, such as excellent resistance to pressure up to 1200 PSI, high customization and the best usability in the market, combined with compatibility with Gamma and E-Beam sterilization up to 60 kGy. This combination guarantees the highest level of safety for high pressure stopcocks, without compromising user friendliness.
The new HP+ stopcock is available in a variety of configurations and HP+ Manifold for Gamma and E-Beam will be available in the future
Read more about Elcam's High Pressure products
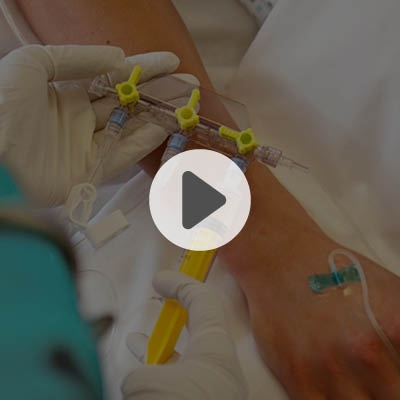
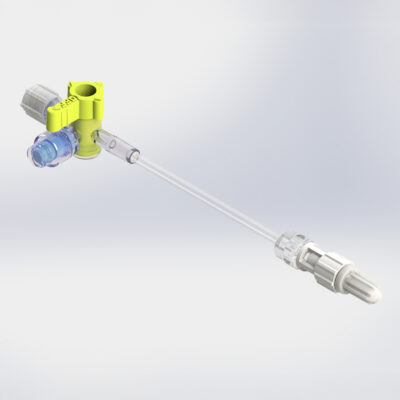
Y-Click
Elcam’s Y-Connector for PTCA applications, is an easy-to-use hemostatic device suitable for both diagnostic and interventional cardiology procedures.
Y-Click provides easy maneuvering during the introduction, use, and withdrawal of diagnostic and interventional devices used in angioplasty procedures such as guide wires, guide catheters, balloons, and stents.
It provides intuitive single hand operation, ease of use, and minimal blood loss which is essential during interventional procedures.
Learn more about Y-Click

Safe2 Rotator™
New in Elcam Medical Italy – recently installed specialized Safe2 Rotator™ (S2R) assembly machine. This new equipment is designed to increase production capacity and meet the growing demand for our products.
Elcam Medical’s Safe2 Rotator™ is a simple yet unique IV therapy connector that sets a new standard for safety and efficiency. Its polyethylene flexible material, 360° rotation, and smooth disconnection make it a superior choice over generic connectors for improving patient as well as medical teams’ safety. The S2R innovative design minimizes patient injury, reduces the risk of infection, and ensures smooth and reliable fluid delivery.
Secure your connections with the Safe2 Rotator™
Visit the S2R product pageEcovadis Rating 2024
Elcam is proud to share that we have achieved a Silver medal in the 2024 EcoVadis sustainability rating. This recognition is a testament to our ongoing efforts to reduce our environmental impact and create a positive social impact. Elcam’s high score of 88th percentile places us in the top 15% of companies in our industry. We are committed to continuing our sustainability journey and making a meaningful difference in the world.

Team News
Michael Segev is Elcam’s new Chief Business Officer (CBO). With 19 years of experience at Elcam, as Manufacturing Engineering Manager for 5 years and for the last 14 years as Chief Technology Officer, Michael brings a deep understanding of our operations and technology with a proven track record of driving innovation and growth. Coupled with his educational background in Mechanical Engineering (B.Sc.) and Business Administration (MBA) and former employment in several start-up companies, Michael is an invaluable member of our executive team. He shared with us: “As the new CBO of Elcam Medical, my vision is to elevate our position as a leading innovator and trusted partner in the medical device industry, by delivering exceptional value to our customers, fostering a culture of collaboration and excellence, and driving sustainable growth through strategic initiatives. My commitment, together with the Marketing and Business Development team, extends beyond just delivering products. We aim to build long-term partnerships”.
Roi Barot is Elcam’s new Territory Manager for Asia, Middle East & Latin America. Roi joined our team 2.5 years ago and worked in the Manufacturing Planning department. Before Joining Elcam he was the Manager of Kibbutz Baram’s Apple Packing factory for 6 years and brings with him abundant managerial experience. His educational background in Political Science and East Asia Studies (B.Sc.) provides him with an important perspective on international business. Roi told us: “I’m thrilled about my new chalenge. I believe in team work and believe that by combining team work and proffesionalism, we can continue to help Elcam grow and thrive”.

Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions

Ortho Summit
14-17 September
Las Vegas, Nevada
Booth 921

Medtec China
25 – 27 September
Shanghai, China
Booth 2D001

Compamed
11-14 November
Düsseldorf, Germany
Hall 8a Booth E28

PDA
22-23 October
Phoenix, Arizona
Booth 409

PODD
28-29 October
Boston, Massachusetts
Table 9
Elcam continuously strives to improve product offerings and supporting services. In this edition of our newsletter, we highlight Elcam’s solutions for ORTHOPEDICS and our state of the art manufacturing capabilities, as well as share updates of Elcam's current situation.
Elcam Stands Strong– Update #8
As we navigate through the seventh month of the conflict in Gaza, we remain hopeful for peaceful times ahead. We want to assure you that despite the ongoing situation, Elcam Medical has maintained a stable routine, ensuring that our operations continue without interruption.
We are pleased to report that all Elcam facilities are operating at full capacity. Our logistics remain secure and on schedule, with no disruptions in our supply chain. The safety and well-being of our employees are paramount, and we have ensured that all necessary measures are in place to keep our teams safe.
In recent months, we have observed a significant increase in personnel presence at our facilities in both Bar’am and Dalton. This return to the workplace, despite the option to work remotely, is a positive indicator of our gradual return to routine operations. This shift underscores our commitment to maintaining the highest standards of production and service.
As part of our ongoing commitment to resilience and strategic growth, we are excited to announce the initiation of a project to add another manufacturing facility in Central America. This expansion is an important layer of our contingency plan, which has already proved its effectiveness. This new facility will enhance our capability to serve you better and ensure even greater reliability of supply.
We are continually monitoring the situation and are prepared to make any adjustments necessary to ensure the safety of our employees and the continuity of our services to you.
Thank you for your continued trust in Elcam Medical. We are committed to keeping you informed and ensuring that our partnership remains strong, even in these challenging times.

New production site in Central America
Elcam remains committed in its dedication to operational excellence and, in accordance with our contingency plan, we are enhancing our strategic initiatives for the future. This entails progressing, as previously communicated, with our plan to establish a new manufacturing facility in the broader vicinity of Central America to further fortify our resilient supply chain.
We are pleased to announce that a decision was reached in late May to designate the Dominican Republic as the country for this endeavor. This location was chosen due to its status as a central hub for medical device manufacturers, enabling us to better serve our customers in North America.
Currently, we are in the process of identifying the precise industrial park within the Dominican Republic. Once this selection is finalized, construction of the facility will begin promptly. Elcam anticipates commencing local production in the third quarter of 2026.
Orthopedics
Elcam has recently entered the world of Orthopedic care which encompasses the diagnosis, treatment, and rehabilitation of different musculoskeletal conditions.
As a long-time provider of solutions for critical care, Elcam is well aware of the importance of high quality treatment and tailor-made solutions and plans to use its vast experience and knowledge to meet the specific needs of orthopedics.
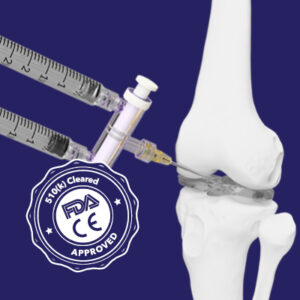
A-TapTM
A-Tap™, Elcam Medical’s innovative solution for intra-articular injections offers precision, efficiency, and enhanced patient comfort.
Research shows that up to 20% of intra articular injections miss the right site, leading to suboptimal treatment and ineffective pain relief. With A-Tap, physicians can ensure that medication is delivered precisely where needed, thanks to the backflow technique and minimal needle movement that verifies and maintains correct placement
Part of Elcam’s launching program is dedicated to working with distributors. See below how one of our distributors is introducing and educating his staff on working with the A-Tap
A-Tap is 510 (k) cleared and CE approved
Learn more
Elcam’s Automation Capabilities
Elcam is a leading manufacturer of top-tier disposable medical devices and accessories tailored for the OEM market. Central to our ability to uphold unparalleled quality is Elcam’s reliance on automated injection molding and assembly processes throughout production.
Cutting-edge tooling, multi-cavity molds, and two-component molding in adaptable injection machines fabricate the necessary components, which are subsequently assembled in Elcam’s fully automated assembly lines. This entire procedure adheres rigorously to stringent quality control measures until the products are prepared for dispatch from our warehouses.
Consequently, every Elcam client can be confident that the medical devices he receives not only meet but exceed the most rigorous standards, ensuring their suitability for application.
Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions

Congreso AEA-SEROD
26-28 June
Barcelona, Spain
Booth 36
(with Enelini Associats)

Medtec China
25 – 27 September
Shanghai, China
Booth 2D001
Elcam continuously strives to improve product offerings and supporting services. In this edition of our newsletter, we highlight Elcam’s solutions for INTENSIVE CARE & ANESTHESIA, In addition, we will share updates of Elcam's current situation and the results of the recent customer satisfaction survey as well as introduce a new team member.
Elcam Stands Strong– Update #7
We are pleased to confirm that our operations in Israel are continuing at full capacity with no disruptions, adhering to the special routine protocols we have established.
As part of our ongoing commitment to operational excellence and in line with the contingency plan previously communicated, we are now strengthening our strategic measures for the future. This includes moving forward with our plan to add a new manufacturing facility in Central America to further boost our robust supply chain. By the end of Q2 2024, we will decide on the location and begin work on the production site.
We want to emphasize our continued close collaboration with Israeli officials and defense forces to ensure the ongoing safety and productivity of our Israeli facilities. Thanks to these partnerships, our dedicated employees and our proactive planning, we have successfully maintained uninterrupted production and supply.
Rest assured, Elcam Medical remains steadfast in our dedication to delivering the high-quality products and reliability you have come to expect from us, even in these challenging times

Intensive care & Anesthesia
As a long-time provider of solutions for intensive care & anesthesia, Elcam is well aware of the importance of high-quality treatment and reliable monitoring in critical care. Professionals in the medical device industry operating in this field require peace of mind when sourcing these devices and a committed partner that understands their needs and problems.
Elcam Medical is your source for the widest variety of Intensive Care & Anesthesia products including our renowned Elcam Stopcocks brand. We partner with our customers to provide high quality, differentiating and innovative solutions along with the everyday devices you need, with special emphasis on maximizing patient and caregiver safety.
Following are a couple of excellent examples from Elcam’s Intensive care & Anesthesia devices
Explore more in the following link
Sense-ITTM (DIPT)
Patient Monitoring in Intensive care and Anesthesia is a mission critical application in which you cannot allow your product, whether an Arterial Line, Central Venous Pressure line, or other pressure monitoring products, to fail.
The Sense-IT™, Elcam’s Disposable Integrated Pressure Transducer (DIPT) is indicated for pressure sensing during clinically invasive procedures. It is the first and only fully integrated pressure transducer. This integration provides reliability and robustness as no other transducer can provide, along with the space saving and ease of use so much needed in Intensive Care Units and Operating Rooms. As in many of Elcam’s products, the Sense-IT™ (DIPT) is also adaptable in dimensions such as tube fitments and cables connectors, to perfectly meet your requirements.
Learn moreMarvelousTM
The Marvelous™ is the first minimal residual volume luer-activated stopcock that was designed by Elcam to increase patient safety and streamline medical procedures. Its unique inner channel effectively reduces dead space and ensures continual flushing, addressing concerns about contamination risks and catheter-related bloodstream infections (CR-BSI) as well as drug residuals and related adverse events. Marvelous simplifies priming and can improve the efficiency of flushing.
Read more
NIP®
Elcam’s NIP® needle-free stand-alone connector can help reduce line contamination and enhance patient and caregiver safety. With a swabbable Luer-activated valve that maintains a fully closed connection throughout use, the NIP® is a safer, more reliable option for patients and caregivers. Plus, with confirmed bacterial barrier protection for 11 days – including seven days with lipid solutions – the NIP® is an ideal solution for ICU and anesthesia needs.
Explore more
Customer Satisfaction Survey
Like every year we have sent you Elcam’s annual customer satisfaction survey in December and have recently finished summarizing its results.
We would like to thank all of you that took the time to answer the questions and help us with Elcam’s consistent efforts to improve its services.
We were happy to see that the efforts we have been investing in recent years in improving our on-time delivery and responsiveness time have resulted in customers’ continued satisfaction. We are aware there are areas in which we still need to improve and we intend to do so in 2024
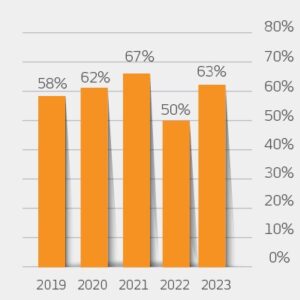
The Net Promoter Score (NPS score) measured annually representing the customers loyalty levels (a positive score is considered good, and an NPS of +50 excellent), improved in 2023 after taking a short plunge in the previous year. Elcam aims to keep scoring above 60 in the following years.

Team News
Bill Anton is our new Sales & Account manager on the Elcam US Team. Bill joined the Elcam US team in January 2024. He has an extensive background in Sales, Product Management, Professional Education and Executive Account Management. Most recently he worked for one of Elcam’s larger US customers for 13 years as a Corporate and Government Account Executive covering large systems on the East Coast. Bill has his Bachelors of Science in Physician Assistant (PA) Studies from Hahnemann University in Philadelphia. He has practiced clinically and spent time working in Emergency Medicine/Trauma Surgery. (Additionally, Bill is a former US Army Combat Medic.) That said, Bill is looking forward to growing Elcam’s market share among the US medical device manufacturers and promoting Elcam’s dynamic new technologies, capabilities, and products.

Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
Medtec Japan
17 – 19 April
Tokyo, Japan
Booth 2308
Elcam continuously strives to improve product offerings and supporting services. In this edition of our newsletter, we highlight ELCAM DRUG DELIVERY DEVICES (E3D), a subsidiary of Elcam Medical that focuses on development and supply of devices for self-administration of injectable drugs. In addition, we share updates of Elcam's current situation and introduce new members of our team.
Elcam Stands Strong– Update #6
Our team at Elcam continues to demonstrate admirable resilience, maintaining our dedicated focus and commitment to ensuring minimal, if any, disruptions. This special working routine has proven its effectiveness not only in our production, deliveries, and logistics but also in seamlessly managing our daily tasks, activities, and ongoing projects.
Our contingency plan has indeed proven to be highly effective, allowing us the sustainability needed for the foreseeable future. This steadfast approach ensures our ability to meet your needs without compromise.
Rest assured, our entire team remains devoted to delivering exceptional service and support to you, our valued customers. We are here to address any queries or requirements you might have.
We deeply appreciate your ongoing trust and partnership during these times.

Elcam Drug Delivery Devices (E3D)
There is a growing demand for devices, which facilitate the injection of parenteral drugs outside of the healthcare environment. This demand is primarily driven by the numerous new biologic drug products – and corresponding biosimilar versions of these biologics- which are used in the treatment of a wide range of long-term therapies that address chronic conditions that require subcutaneous injections and can be self-administered.
E3D is focused on addressing the need for development, manufacturing and supply of drug delivery devices for self-administration of injectable drugs in non-clinical environments. E3D’s broad portfolio encompasses devices, which are suitable for drugs in a range of primary containers, including auto-injectors, on-body delivery systems and smart devices with wireless connectivity and data logging features.
Following are a couple of excellent examples from E3D’s device portfolio
Explore more in the following link

Flexi-Q eMU-P
E3D’s Flexi-Q eMU-P is an electro-mechanical multi-use auto-injector system for drugs in pre-filled syringes. It was developed following extensive usability tests involving patients from diverse groups with a variety of conditions and injection-related challenges. The eMU-P is comprised of a reusable Smart Device and a single-use Cassette which incorporates a drug-filled syringe. It was specifically designed for ease of use and patient safety with clear, real-time guidance and confirmation on correct use.
Since the drugs are self-administered at home, a key consideration is to be able to verify that prescribed medications are being administered at the correct dosage and at the right time; Flexi-Q eMU-P has a unique wireless connectivity and data capture capability that can enable physicians to remotely monitor adherence to treatment. Other features include automatic verification of the drug’s temperature and expiry date.
Learn moreFlexi-Q mMU
E3D’s Flexi-Q mMU is a mechanical multi-use auto-injector system that integrates a reusable Device with a single-use Cassette incorporating a drug-filled syringe. .
With just one additional user-handling step compared to a traditional single-use auto-injector, the mMU offers all of the convenience, ease-of-use of single-use devices -including needle shielding, automated injection and automatic needle safety.
Its reusable, user-friendly, and cost-effective design positions Flexi-Q mMU as an optimal choice for long-term therapies for chronic conditions, addressing not only patient needs but also aligning with healthcare sustainability goals by reducing waste, minimizing storage volume and lowering costs.
Read more
Team News
Rhonda Cohen joined the Elcam US team in October. Rhonda’s background is in sales and account management. Most recently, she worked for one of Elcam’s larger US customers as the Vascular Account Executive in New Jersey and the areas of Pennsylvania and New York State. She has a bachelor’s degree from Barnard College and an MBA from the Garvin School of International Management. Rhonda shared with us: “I’m looking forward to growing Elcam’s market share among US medical device manufacturers and promoting Elcam’s capabilities for developing new technologies”.

Emily Maestro Gonzales joined the Customer Service team in Israel in August, where she is in charge of orders and shipments from USA, South America and Asia. She was part of the Customer Service team in 2019 and since then has fulfilled different managerial roles in the agriculture industry – in the plantations and packing factory of Kibbutz Baram and as a veterinarian assistant. Emily shared: “I’m very happy to have the opportunity to rejoin Elcam’s team. I look forward to continue working with Elcam customers, to learn and gain more experience in providing the best solutions to their needs”.

The Elcam team wishes you and your loved ones a joyful holiday season and a peaceful, healthy and prosperous year in 2024
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
Pharmapack
24 – 25 January
Paris, France
Hall 7.2 | Booth H14
MD&M West
6-8 February
Anaheim, California
Booth 2445
Elcam continuously strives to improve product offerings and support services. In this newsletter edition, we highlight the ELCAM DEVICES sub-brand that is a major part of our business, focus on our latest products and news in this area, and share our vision for digital transformation.
Elcam Devices™
The Elcam Devices product line represents Elcam’s commitment to the OEM market and our obligation to remain innovative and current in providing differentiating solutions for safe and effective flow control needs.
This sub-brand includes 3 main sections of our product lines – Patient Monitoring, Medication Delivery, and Interventional Procedures. Each of these sections includes dedicated devices that were developed to create solutions to real flow control problems with an emphasis on patients’ and care givers’ safety.
The New A-Tap™ for orthopedic procedures, the Y-Click for interventional cardiology procedures, and the Safeport™ manifold for anesthesia and ICU applications are all part of this brand and are excellent examples.

A-Tap™ – Precision in Intra-articular Injections
Elcam Medical designed the new an innovative A-Tap™ to facilitate intra-articular injections. This medical solution optimizes injection procedures, focusing on reducing procedure time, enhancing accuracy, and minimizing needle movement.
The A-Tap™ enhances treatment precision and efficacy with by confirming needle tip position with aspiration, which supports best practice recommendations. Enabling two procedures with one needlestick and minimizing needle movement, it can greatly improve both practitioners’ and patients’ treatment experience.
The A-Tap is now CE approved and 510(k) cleared and available for sale
Explore more in the following link

Y-Click – Hemostatic PTCA Valve
Cathlab procedures require working areas with minimum bleeding. Elcam’s Y-Click connector was designed in collaboration with interventional experts with this need in mind – to provide a hemostatic solution for percutaneous transluminal coronary angioplasty (PTCA) procedures and other diagnostic and interventional applications.
Its most unique advantages – three working positions, self-closing mechanism and one-handed operation, make the Y-Click connector an easy-to-use hemostatic device that improves physicians’ performances

Safeport™ Manifold
Induction of anesthesia during operation is involved with enormous responsibility and requires multiple skills and focus. Elcam’s SafePort™ is a unique function manifold with patented dual flow option innovative side-port valves that can simplify the anesthesiologists’ work during procedures.
The SafePort is now available with a new improved design in which the handles are injected using 2C molding technology, guaranteeing improved accuracy and reliability.
SafePort™ is also available as a closed system with integrated Swabbable Luer Activated Valves, for minimizing leakages and exposure to air and contamination
Read more
Digital transformation
Digital transformation is all around us affecting many facets of life and business. Elcam is navigating these changes with an ongoing commitment to advancing through digital transformation, aiming to enhance work efficiency, simplify procedures, and boost our competitive edge, all driven by technology.
These are some of the steps Elcam will continue to vigorously promote to achieve this goal:
- Reducing manual work by means of incorporating automatic procedures and the use of technologies that enable work simplicity and efficiency.
- Combining data technologies and AI to reach decisions based on accurate and reliable data while maintaining the data and sources transparency and consensus.
- Introducing reliable, advanced, and automatic systems to improve procedures’ quality and ensure dependable, efficient, and qualitative work.
- Upgrading capabilities of information retention, knowledge, and employees by investing in technological processes, employee training, and skills adaptation to the new working world.
- Strengthening the culture of ongoing change and innovation to promote new standpoints and creative solutions.
With these ongoing steps Elcam aims to create an organizational climate that will provide our employees with an advanced and interesting work environment that fosters personal and organizational growth.

Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
ICRS 2023 | 17th ICRS World Congress
9-12 September
Barcelona, Spain
Stand 13
PDA Universe of Pre-filled Syringes
17-18 October
Gothenburg, Sweden
Booth 63
Compamed
13 – 16 November
Duesseldorf, Germany
Hall 8a Booth E28
Elcam continuously strives to improve product offerings and supporting services. In this newsletter edition we highlight the ELCAM STOPCOCKS sub-brand that is a major part of our business and focus on our most advanced and innovative stopcocks. In addition, we are happy to introduce new team members and discuss the results of our annual customer satisfaction survey.
Elcam Stopcocks
Elcam Stopcocks™
Elcam Medical is a world leader in OEM stopcocks, offering the widest range of Stopcocks and Manifolds for all fluid control related applications. Elcam Stopcocks™ is our sub brand enhancing our leadership in this field and over 40 years of experience and expertise in developing and manufacturing medical disposable stopcocks. Professionals in the area of medical devices know that when searching for a stopcock – a critical component of the final product – they need a supplier who can offer best fit to all varieties and applications, a product that will work flawlessly at all times and, most importantly, uncompromising regulatory, quality, engineering and customer support to come with it. Elcam’s leadership in stopcocks ensures the benefits professionals value so much: broadest portfolio, innovation, seamless integration, highest quality standards and all-around support.
Elcam believes in continuous enhancements of product features and Elcam’s Stopcock Road Map is a great example of this promise. This program was established 15 years ago in order to continue and develop advanced stopcock designs that better serve the needs of our customers, increase treatment safety while keeping handling and use easy and simple for care givers.
The Marvelous™, Closed and Safe2 Rotator™ stopcocks are part of the products that were developed under this this program and serve as excellent examples of this activity.
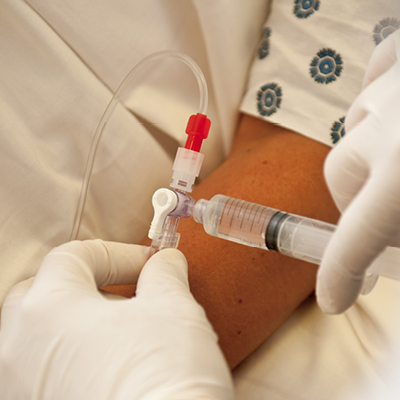
Marvelous™
Marvelous™ is the first minimal residual volume luer-activated stopcock designed to save time and increase safety with two main features: self-flushing and closed & swabbable luer-activated valve (LAV).
The Marvelous two main features both contribute to patient and caregiver safety and workflow improvement:
The LAV serves as a bacterial barrier, allowing access to the line without opening it and producing a closed system and needle-free injection site.
The fluid flow around the handle creates a unique “circumferential channel” that reaches the entire internal volume of the valve. Whether a drug or blood, the internal volume is constantly flushed by the in-line flow providing greater protection against blood clotting, bacterial colonization and residual drugs related complications.
Furthermore, the Marvelous improves work flow, saves precious time and reduces costs
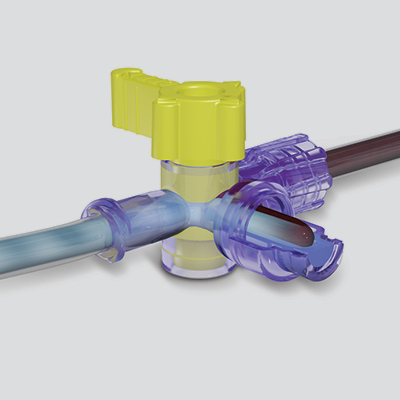
Closed Swabbable Stopcock
Closed Swabbable Stopcock
A Closed Stopcock, integrated with a Swabbable Luer Activated Valve, allowing for maintenance of a fully closed system through the entire use of the stopcock, as well as a needle free work environment. Elcam’s Closed stopcock enhances patient safety by providing full barrier protection against microbial and air ingress, thereby reducing the risks of infections and air embolism. This stopcock also improves caregiver safety by preventing needle stick injuries and avoiding drug and blood spills. It improves workflow by replacing the need to add a cap or LAV on a stopcock and eliminates the need for additional adaptors or cannulas
Read more
Safe2 Rotator™ Stopcock
S2R Stopcock
Elcam’s Safe2 Rotator™(S2R) Stopcock with the spinning lock feature provides a small yet significant contribution to the efforts of keeping medical teams and patients safe. It was designed to ensure secured connection along the entire treatment so critical to safe and effective IV therapy. The S2R Stopcock features a 360º rotational flexibility that can prevent accidental disconnections, reduce tube kinks and twists in the tubing set, facilitate convenient approach to injection and sampling ports, prevent leakage due to over-tightening and more
Learn more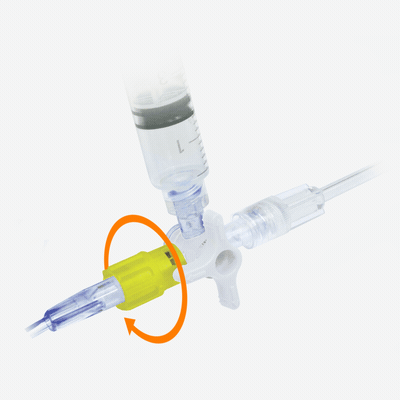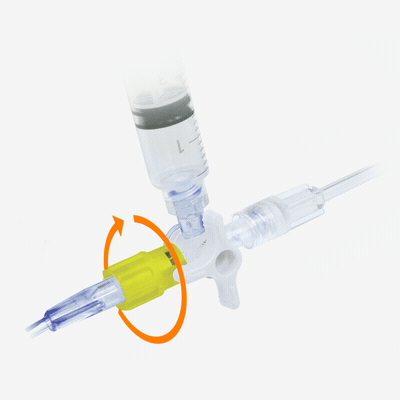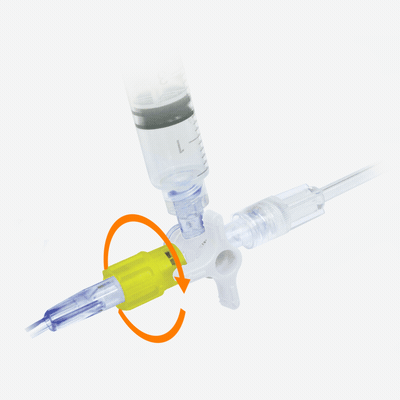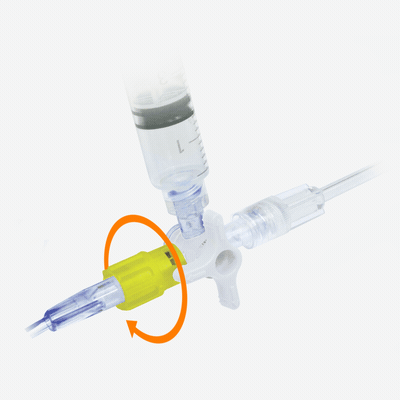
Team News
Team News
Shivani Shivkumar is our new Customer Service for Europe.
Shivani joined our team 4 months ago. She is originally from India and relocated to Israel at the end of 2020 to build her family in Baram.
Shivani has a Bachelor degree in Science as well as in Communication and Marketing, and she previously worked as a business development manager for an advertising agency in India.
Shivani told us: “I am in admiration of working in an environment that functions as a community, I want to bring in my people skills and calmness to build a harmonious relationship with Elcam’s clientele. I’m really happy to be able to contribute to Elcam’s growth”
Jessica Miller joined our US team 2 months ago as a Customer Service Specialist. Her background is in office administration, customer service, inventory and purchasing with over 30 years’ experience and her last position was Service Administrator at Winter Scale & Equipment.
Jessica shared: “I look forward to growing in my position with my skills and experience and being a part of Elcam’s continued growth and success. I am excited to have joined such a great and amazing team at Elcam”

Customer Satisfaction Survey
Customer Satisfaction Survey
Like every year we have sent you Elcam’s annual customer satisfaction survey in December and have recently finished summarizing its results.
We would like to thank all of you that took the time to answer the questions and help us with Elcam’s consistent efforts to improve its services.
This year we received lower results than in previous years in terms of responsiveness time and on time delivery for both long lead-time and not meeting delivery dates. Elcam is well aware of this situation which is mainly related to major changes and peaks in demand as well as to the worldwide logistic crisis.
On the other hand, Elcam continues to score very high in terms of product quality and our customers continue to appreciate Elcam’s quality regardless their satisfactions in other aspects of our services.
Our Net Promoter Score (NPS score) measured annually representing the customers loyalty levels (a positive score is considered good, and an NPS of +50 excellent), respectively decreased in 2022 to 50% but is still in the positive range.
Amir Bohadana, Executive Sales Director, elaborated: “Your important feedback together with the challenges we faced during 2022, have increased our motivation to take the necessary actions to keep our services at top level. This need was acknowledged by Elcam’s management and became one of our main topics in the 2023 plan”.

Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
Medtec China
1-3 June
Shanghai, China
Booth B1- H003
Elcam maximizes efforts to provide qualitative customized solutions to our customers greatest needs and challenges. With a commitment to provide an excellent customer experience, Elcam constantly thinks of new ways to improve the services we offer our customers whether through our product innovation or by upgrading processes and services.
Joint Validation & Verification
Joint Validation & Verification
Over the past few years, it has become evident that medical device companies are looking for ways to expedite approval processes of new designs. A highly effective process for Joint Validation & Verification (V&V) activities is a customized service offered by Elcam in order to meet this need and remove the burden from the medical device provider. The process complies with ISO 13845 standard requirements.
It is well known that when a medical device manufacturer sources certain critical components from a supplier, the component must comply with specifications and be validated for a certain application. This validation is a documented guarantee that a product can be manufactured reliably and repeatedly to satisfy a predetermined level of quality.
Many companies invest meticulous efforts and valuable time in product or component acceptance activities. The traditional V&V approach in sourcing a new or substitute component is usually done in series of stages on both sides – component manufacturer and OEM.
A mutual V&V program will reduce the work load of the medical device provider. This service that is offered by Elcam can significantly accelerate the approval process and time to market of the end product in addition to saving the provider a significant amount of resources.
Download our whitepaper
.f_p{ transform: translateX(-80px);}
@media only screen and (max-width: 750px){
.f_p{ transform: translateX(0);}
}
Elcam strives to enhance patients’ and medical teams’ safety and treatment experience. Following you will find two excellent examples of new and improved products that were designed with these intentions in mind.
A-Tap™
The new and innovative A-Tap™ by Elcam was designed to facilitate intra-articular injections by allowing aspiration and injection to be done with the same device, thereby reducing the number of steps for practitioners and improving the patient treatment experience.
“A-Tap™ makes a cumbersome procedure easier for both the practitioner and the patient” says Dr. Michael Wilmink, MD, Orthopedic Surgeons
By allowing aspiration and injection to be carried out with the same device, the A-Tap™ cuts down the number of steps for practitioners and reduces procedure time.
Enabling two procedures with one needlestick and minimizing needle movement, this new device can greatly improve the patients’ treatment experience.
Furthermore, the A-Tap™ enhances treatment accuracy and efficacy with the confirmation of needle tip position by aspiration, which supports best practice recommendations (ACSM’s Sports Medicine: A Comprehensive Review 2012 I Francis G. O’Connor I pg.126).
Watch the video
SafePort™ Manifold
SafePort™ Manifold
The SafePort™ is an improved function manifold with patented dual flow option innovative side-port valves designed to improve safety in anesthesia and ICU applications. The two flow options are controlled by a simple 900 turn of the handle: Accessible for two-way free flow or one way pressure activation.
The SafePort is now available with a new improved design in which the handles are injected using a new technology of 2C molding which guarantees improved accuracy and reliability.
As with the previous design , the new SafePort™ is also available as a closed system with integrated Swabbable Luer Activated Valves, for minimizing leakages and exposure to air and contamination
Read more
Team news
Team news
Tomer Gil has replaced David Landspurg as General Manager of Elcam Medical Inc. Tomer has been part of the Elcam team for 20 years and recently relocated to the US to empower the US business development activities, a role which he will continue to uphold, together with managing our US office in Hackensack New Jersy. Join us in wishing him a lot of success in his new position.

Yolanda Roman has joined the Elcam Medical Inc. team in New Jersey on July 2022 in the role of Customer Service Specialist. Her background is in Customer Service, Management and Purchasing with over 30 years of experience.
Yolanda told us: “I am excited to have been given this opportunity to work here at Elcam with a very gifted and welcoming team. I look forward to growing in the position and bringing my skill set from my years of experience in Customer Service”.
Welcome Yolanda!

All of us here in Elcam wish you and your loved ones a joyful holiday season and a happy, healthy and prosperous new year 2023
Best regards,
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
Pharmapack
1-2 February
Paris, France
Hall 7.2 Booth F71
MD&M West
7-9 February
Anaheim, California
Booth 2445
Elcam is excited to introduce the company's core value chosen for this quarter – CARE. See how our commitment to caring is expressed in Elcam's latest and ongoing activities – sustainability and corporate responsibility, new assembly capabilities, new website and the measures we are taking to minimize the effects of the global logistics crisis.
Elcam CARE
Elcam CARE
Elcam’s core values are Care, Consistency, Quality and Growth. Each quarterly newsletter during 2021 focuses on one company value and shows how it is implemented and expressed within the organization. The chosen value for this newsletter is CARE.
CARE has been one of Elcam’s core values for many years spanning our entire operation and state of mind, from patient care to caring for one another, care is at the heart of everything we do. It is working as one team, regardless of where we are in the world. It means respecting all people and celebrating a diverse workforce where everyone is treated fairly and honestly. It is caring for the society and environment in which we live in – for our communities, our planet, and for future generations.
In this newsletter we will focus on how the value of caring is expressed in Elcam’s activities.

Sustainability
Sustainability
At Elcam we believe that sustainability is no longer a buzz word but a way of living and caring for all that surrounds us. Igal Kohn, CEO Shared: “We are committed to maintaining good corporate citizenship with a holistic attitude that provides balance between providing the best clinical outcomes for patients and caregivers, and maintaining a positive impact on all areas comprising Elcam, including workforce, environment, economics and community.”
Social responsibility for us means to promote diversity and inclusion between our employees who represent many minority groups and to provide equal non-discriminating working opportunities regardless of origin, sex or age. Through our environmental responsible actions Elcam aims to reduce its environmental footprint in order to leave a healthier planet for future generations. These actions include recycling, energy preservation, environmentally friendly energy generation using trigeneration and solar energy. Being a business responsible company is part of our environmental, social and governance (ESG) responsibilities, and Elcam is obligated to high standards of ethics and business conduct, operating both internally and externally in a fair and non-exploitive manner. Elcam is also committed to rules and regulations concerning cybersecurity and Data protection.
Our Corporate Responsibility
New Assembly Capabilities
New Assembly Capabilities
Elcam is always striving to gain new capabilities in all facets of the organization in order to provide better service to our customers. In the last few years, one of Elcam’s main endeavors has been to gain new capabilities in assembly technologies introducing modern and more sophisticated assembly machines.
For example, the new tube assembly machine in Elcam Medical Italy (EMIT) that assembles stopcocks manufactured in Elcam Israel with tubes and components manufactured in EMIT. Gianluca Menghi, General Manager EMIT, elaborated: “With this new machine, we aim to design and develop new sets according to the different needs and requirements of our customers. It is very versatile allowing assembly of both straight and coiled extension lines, with different types of connectors, activating or disactivating stations, when necessary for a specific product design. The machine performs 100% flow test to assure that no obstruction is present. Moreover, the machine includes many control systems that verify during the process if parts were correctly assembled while each station is able to scrap a faulty product through a ramp”.
Another great example is two new assembly machines that are currently being added to the transducer line in Elcam Israel all in an effort to improve production time and capacity as well as diversify our product offering. One machine will combine the work of 2 separate older version machines including lase drilling, and also carry out flow tests. The second will perform fully automatic soldering, 100% in-production tests to assure electrical and leakage conditions, and will have much higher production rate.
New Website
New Website
The new Elcam website that was recently launched, was developed for easier and simpler navigation with several drop-down menus that enable the viewers to quickly reach their point of interest. No efforts were spared to improve the site’s user interface, as part of Elcam’s philosophy of providing the best customer experience. In the new website you can find our products as well as our advantages in terms of company mission, partnering, corporate responsibility, global presence and more.
Our New Look
Covid Logistics
Covid Logistics
At Elcam Medical, customer service is paramount and we pride ourselves on meeting our customer’s needs. It’s no secret that the pandemic and the current global logistics crisis has disrupted all of our businesses in some way or the other. Globally, there continues to be massive delays due to container availability, sea shipping and port backlog, reduction in available air cargo routes and limited trucks & container chassis. That coupled with limitations in human resource such as port workers, truck drivers and warehouse personnel add up to a very significant challenge.
Amir Bohadana, Chief Sales Officer, specified: ” With no near-term end to this crisis in sight, Elcam is working internally, working with suppliers and working with our customers to minimize impact as best possible through a variety of methods: workers vaccinations, expedited deliveries as best possible, automation in warehouse facilities, additional warehouses and safety stocks of both products and raw materials, as well as exploring new potential alternate sources of raw material supply”.
Covid Updates
On behalf of us all here at Elcam, I would like to wish you and your families a happy, successful and most of all healthy New Year 2022
Igal (Guli) Kohn, CEO and General Manager
Exhibitions
MD&M West
12-14 April
Anaheim, California, USA
Booth 2445